- Title
-
The pitx2 homeobox protein is required early for endoderm formation and nodal signaling
- Authors
- Faucourt, M., Houliston, E., Besnardeau, L., Kimelman, D., and Lepage, T.
- Source
- Full text @ Dev. Biol.
|
Early expression of pitx2 transcripts during Xenopus development. (A) Deduced N-terminal sequences of three Pitx2 isoforms. The sequence common to the different Pitx2 isoforms is in green. The junction between the variable and the common regions coincides with the beginning of exon 5 in pitx2 (see Schweickert et al., 2000, for the genomic organization of the pitx2 locus). (B) Northern blot of total RNA prepared at the indicated stages. The blot was probed with an antisense RNA probe derived from the 3′ UTR of the cDNA. Similar results were obtained with a probe containing the coding region. (C) RT-PCR analysis showing that in Xenopus, pitx2b, and pitx2d are expressed maternally and zygotically while pitx2c is expressed only after MBT. RT-PCR was performed with total RNA prepared at the indicated stages. (D) Expression of pitx2 in early Xenopus gastrulae (st 101/2) analyzed by dissection and RT-PCR. pitx2b and pitx2c are expressed predominantly in the vegetal and marginal regions while pitx2d is expressed uniformly. Material from a single embryo was used for the 1RT control. Five embryos were used for the animal, vegetal, and equatorial explants and two for the dorsal and ventral fragments. (E–H) pitx2 in situ hybridizations showing an early broad expression at the blastula stage and expression in the dorsal mesoderm and endoderm at the gastrula stage. In situ hybridizations of bisected embryos are shown in E and G and of whole embryos in F and H. (E) Side view showing ubiquitous expression of pitx2 at the late blastula stage (stage 93/4). Note the punctate staining in the vegetal hemisphere. (F) Midgastrula (stage 11) vegetal view, dorsal side at top. (G) Lateral view, dorsal side on the right. pitx2 transcripts are transiently enriched in the involuting dorsal mesoderm. A slight dorsal–ventral gradient of pitx2 transcripts is apparent in the ectoderm, mesoderm, and, to a lesser extent, endoderm. (H) Cleared neurula (stage 15). The most prominent expression of pitx2 is in the anterior ectoderm and prechordal mesoderm. |
|
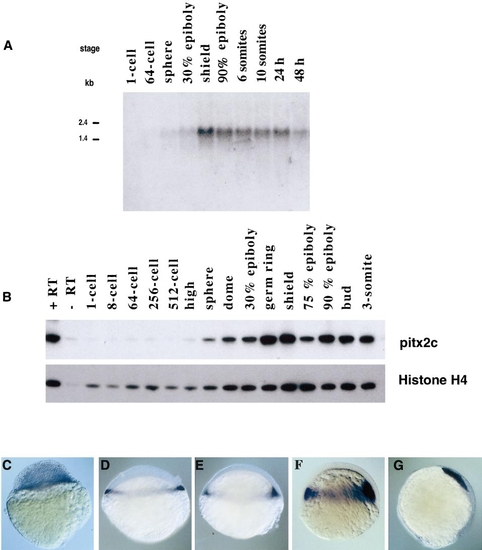
Early expression of pitx2 transcripts during zebrafish development. (A) Northern blot of total RNA prepared at the indicated stages. The blot was probed with an antisense RNA probe corresponding to the whole zpitx2c cDNA. (B) RT-PCR analysis showing that pitx2c transcripts start to accumulate at the onset of zygotic transcription. (C–G) Whole-mount in situ hybridization with a probe recognizing all the pitx2 isoforms showing expression of pitx2 first in the presumptive mesendoderm and then in prechordal mesoderm. (C) Sphere, (D) 30% epiboly, (E) germ ring, (F) shield, (G) 75% epiboly. |
|
pitx2 expression in Xenopus is activated in response to Nodal. (A) Animal cap assay showing induction of pitx2 expression in response to Xnr-1 overexpression. Embryos at the four-cell stage were microinjected at the animal pole in each blastomere with 50 pg of Xnr-1 mRNA. Animal caps were removed at stage 7, treated continuously with 10 μg/ml cycloheximide, and analyzed at stage 9 in parallel with untreated control caps for the expression of Xbra, goosecoid, pitx2b, pitx2c, and histone H4. While Xnr-1 induces Xbra and goosecoid expression in absence of protein synthesis, treatment with cycloheximide prevents the strong activation of pitx2c and pitx2b expression. |
|
pitx2 expression is disrupted very early in zebrafish one-eyed pinhead (oep) mutant embryos. pitx2 expression in wild-type (A–E) and zygotic oepz1 mutant embryos (F–J). (A–F) 30% epiboly (4.7 h), (B, G) germ ring (5.7 h), (C, H) shield (6 h), (D, I) 75% epiboly (8 h), (E, J) bud (10 h). Note that only a weak pitx2 expression is initiated and that pitx2 transcripts rapidly disappear when the Nodal signaling pathway is inactive. |
|
The response of Xenopus animal caps to microinjection of pitx2 mRNA partially mimics the response to Xnr-1. Embryos at the eight-cell stage were microinjected at the animal pole in each blastomere with 25, 75, or 225 pg of pitx2c, pitx2b, or Xnr-1 mRNA. Animal caps were removed at stage 9 and analyzed at stage 11 by RT-PCR for expression of the marker genes indicated. 2RT, control without reverse transcription. pitx2 dose-dependently induces goosecoid, sox17β, cerberus, and antivin but not Xbra or chordin. These results are representative of four independent experiments. |
|
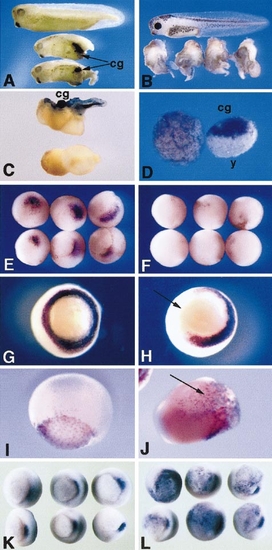
Effects of overexpression of pitx2. (A) Induction of ectopic cement gland and mesenchymal tissue by microinjection of pitx2 RNA into ventral blastomeres. Uninjected stage 34 embryo (top) and two siblings injected with 300 pg of pitx2b RNA into one ventral cell at the four-cell stage. Lumps of tissue containing pigmented and secretory cement gland tissue (cg) are visible in ventral/posterior regions. (B) Stage 41 embryos injected with 200 pg of pitx2b into the two dorsal vegetal cells at the eight-cell stage (uninjected embryo at top). Anterior and dorsal axial structures are absent or greatly reduced, while some additional tail-like structures have formed. (C) Embryos exposed to ultraviolet irradiation at the one-cell stage. Injection of 200 pg of pitx2c RNA into one cell at the four-cell stage induced formation of a cement gland and tail structures but did not rescue dorsal axial structures. The lower embryo was irradiated but not injected. (D) Animal cap explants prepared from stage 9 embryos and cultured until stage 20. The cap on the right was prepared from an embryo injected at the four-cell stage with 50 pg of pitx2c RNA into the animal pole of each blastomere. Extensive cement gland tissue was induced by pitx2c, and a mass of large, yolky, nonadhesive cells (y) is also present. A control explant, which has developed epithelial characteristics, is shown on the left. (E–L) pitx2b RNA (200 pg) was injected into one or two blastomeres at the four- or eight-cell stage and embryos were fixed for whole-mount in situ hybridization at stage 10.5 or 11. (E, F) chordin expression in the organizer region of uninjected (E) and pitx2b-injected (F) embryos at stage 10.5. chordin expression is strongly reduced following injection of pitx2 into the two dorsal vegetal blastomeres at the eight-cell stage. (G, H) Vegetal views of xbra expression in the presumptive mesoderm of an uninjected (G) and pitx2b-injected (H) embryo at stage 10.5. Following injection of pitx2b RNA into the two dorsal vegetal blastomeres at the eight-cell stage, half the Xbra expression has been abolished. (I, J) Lateral views of sox-17β expression in the endoderm of an uninjected (I) and a pitx2-injected (J) embryo at stage 11. sox-17β is expressed ectopically following injection of pitx2 into one blastomere at the four-cell stage. (K, L) Vegetal views of goosecoid expression in uninjected embryo (K) or following injection of pitx2 RNA into the two ventral vegetal blastomeres at the eight-cell stage. Ectopic patches of goosecoid expression are visible in the vegetal and marginal regions. |
|
pitx2-EnR RNA disrupts dorsoanterior development and prevents expression of many endoderm markers. (A–C) Phenotypes caused by injection of 100 pg of the EnR-pitx2 RNA into the two dorsal vegetal cells at the eight-cell stage, including microcephaly, reduction of the size of the cement gland, and mild cyclopia (A); mild cyclopia, shortening of the axis, and spinibifida (B); and severe anterior and posterior truncations and presence of a collapsed blastocoele (b) (C). Uninjected embryos are included at the tops of A–C. (D) Embryos injected radially with 50 pg of the EnR-pitx2 RNA into each vegetal cell at the eight-cell stage and cultured until stage 27. No epiboly has occurred, gastrulation was prevented, and no dorsal or anterior structures have developed. (E–L) EnR-pitx2 RNA was injected into various blastomeres at the four- or eight-cell stage and embryos were fixed for in situ hybridization at stage 10.5–11.5. (E) Xbra expression at stage 10.5 extinguished (arrow) in half the embryo following injection of 50 pg of EnR-pitx2 RNA into two vegetal cells at the eight-cell stage (compare with Fig. 2F). (F) Similar injection with lineage tracing of the injected blastomere. Note the presence of cells that do not express Xbra and do not contain the lineage label (arrow). (G) Bisected uninjected stage 11.5 embryos showing cerberus expression throughout the endoderm, concentrated on the dorsal side. (H) Expression of cerberus is greatly reduced in siblings injected with 50 pg of EnR-pitx2 RNA into the four vegetal cells at the eight-cell stage. (I) Bisected uninjected stage 10.5 embryos showing sox-17β expression throughout the endoderm. (J) Siblings injected with 50 pg of EnR-pitx2 RNA into the four vegetal cells at the eight-cell stage showing greatly reduced expression of sox-17β. (K) Bisected uninjected stage 11 embryo showing expression of sox-17β throughout the endoderm. (L) A sibling injected with 50 pg of EnR-pitx2 mRNA into two vegetal cells at the eight-cell stage in which expression of sox-17β was abolished in half of the endoderm territory. |
|
EnR-pitx2 interferes with specification of the mesendoderm. Embryos were injected vegetally into each blastomere at the four-cell stage with 50, 100, or 200 pg of EnR-pitx2 mRNA, collected at stage 11, and analyzed by RT-PCR for the expression of sox-17β, mixer, GATA-4, and edd (panendodermal markers); dkk-1 and cerberus (anterior endomesoderm markers); Xbra (general mesoderm); chordin (dorsal mesoderm); Xanf-1 and Xanf-2 (ectodermal markers at this stage); siamois and Xnr-1 (dorsal endoderm); and histone H4 (loading control). |
|
Change in the fate of vegetal blastomeres injected with low doses of EnR-pitx2 and reversal by wild-type pitx2 mRNA. Embryos were injected into one ventral vegetal blastomere at the eight-cell stage with β-Gal RNA alone (A, D), β-Gal plus 75 pg EnR-pitx2 RNA (B, E), β-Gal plus 200 pg wt pitx2 RNA (C), or β-Gal plus 75 pg EnR-pitx2 RNA plus 200 pg of wt pitx2 RNA (F) and processed for detection of the lineage label around stage 34. The shift in position of the progeny of the injected cells caused by EnR-pitx2 is reversed by wild-type pitx2. (D, E) Histological sections from equivalent posterior positions through embryos injected with β-Gal RNA (D) or with EnR-pitx2 RNA (E). Note that while in the control embryo β-Gal-positive cells populate mesodermal and endodermal structures, β-Gal-positive cells are absent from the endoderm in the EnR-pitx2-injected embryo. |
|
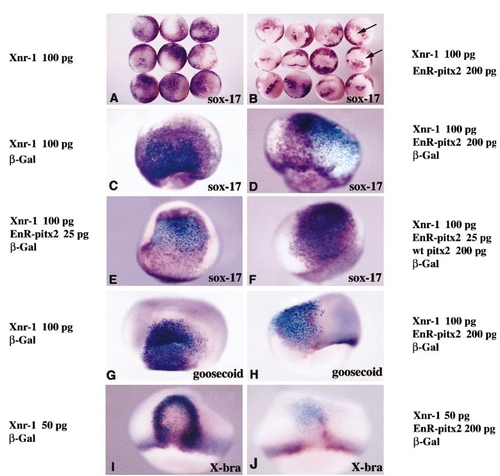
EnR-pitx2 RNA blocks expression of sox-17β, goosecoid, and Xbra in response to Xnr-1. (A, B) Stage 11 embryos injected in two blastomeres at the four-cell stage with 100 pg of Xnr-1 RNA alone or together with 200 pg of EnR-pitx2 RNA. (A) Ectopic sox-17β (purple) induced by Xnr-1 (animal view). (B) Siblings in which 200 pg of EnR-pitx2 RNA was co-injected. Ectopic sox-17β transcripts are seen surrounding sharply delimited patches of unstained cells (arrows). (C, D) Lateral views of embryos from a similar experiment but β-Gal mRNA was included in the RNA mixture to allow lineage tracing (blue). (C) Injection of Xnr-1 alone induced sox-17β expression both in the progeny of injected cells and in surrounding cells. (D) EnR-pitx2 mRNA blocked cell-autonomously the induction of sox-17β. (E, F) Rescue of sox-17β expression by wild-type pitx2 RNA. (E) Cell-autonomous inhibition of induction caused by co-injecting 25 pg of EnR-pitx2 RNA with 100 pg of Xnr-1 into one blastomere at the four-cell stage. (F) Reversal of the effect by co-injection of 25 pg of EnR-pitx2 RNA, 100 pg of Xnr-1, and 200 pg of wild-type pitx2 RNA. Expression of sox-17β in the clone of injected cells is rescued by co-injection of wild-type pitx2 RNA. (G) Ectopic goosecoid induced by Xnr-1 mRNA. (H) Sibling in which 200 pg of EnR-pitx2 RNA was co-injected. Lineage tracing of co-injected β-Gal RNA (blue) shows that ectopic goosecoid is not induced in the progeny of cells overexpressing Xnr-1 when the EnR-pitx2 RNA is present. (I) Ectopic Xbra induced at the periphery of the clone after injection of 50 pg of Xnr-1 into one blastomere at the four-cell stage. The same dose caused induction of sox-17β in the center of the clone (not shown). (J) Almost complete inhibition of Xbra induction at the border of the marked clone after co-injection of 50 pg of Xnr-1 and 200 pg of EnR-pitx2 mRNA. |
Reprinted from Developmental Biology, 229(2), Faucourt, M., Houliston, E., Besnardeau, L., Kimelman, D., and Lepage, T., The pitx2 homeobox protein is required early for endoderm formation and nodal signaling, 287-306, Copyright (2001) with permission from Elsevier. Full text @ Dev. Biol.