- Title
-
CD44a functions as a regulator of p53 signaling, apoptosis and autophagy in the antibacterial immune response
- Authors
- Cao, L., Fang, H., Yan, D., Wu, X.M., Zhang, J., Chang, M.X.
- Source
- Full text @ Commun Biol
|
|
|
|
|
|
|
|
|
|
|
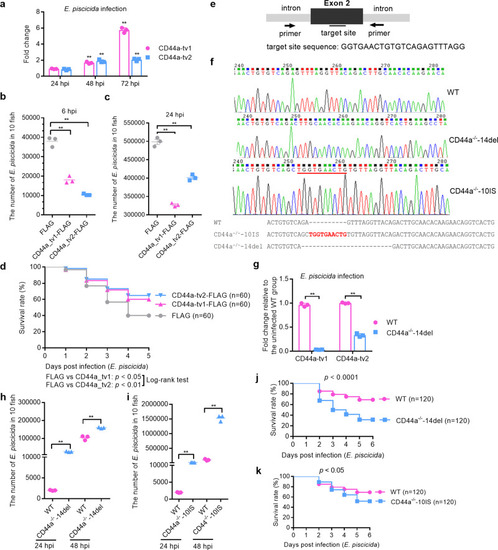
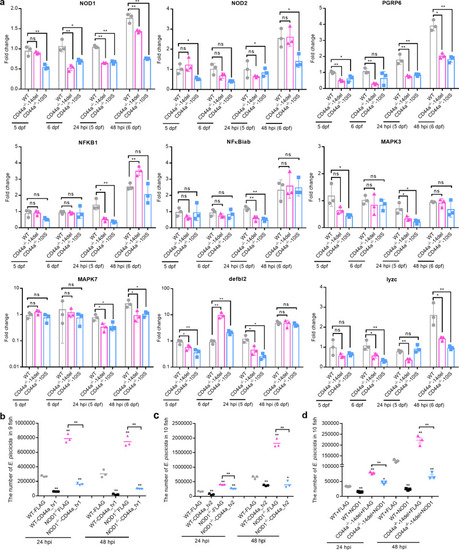
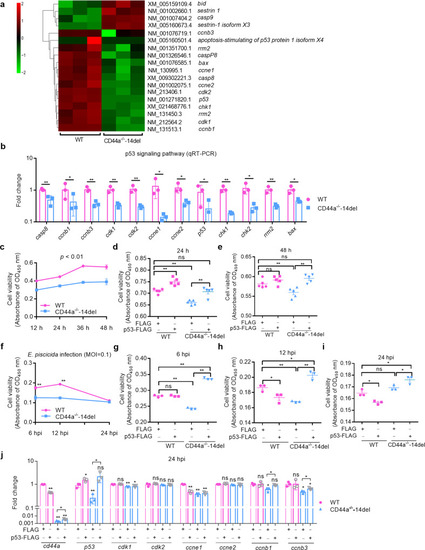
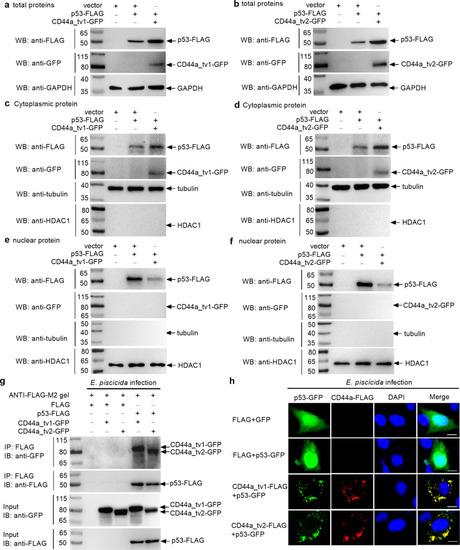
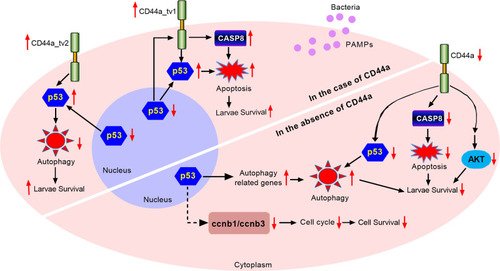
The effects of zebrafish CD44a_tv1 ( |
|
|
|
|
|
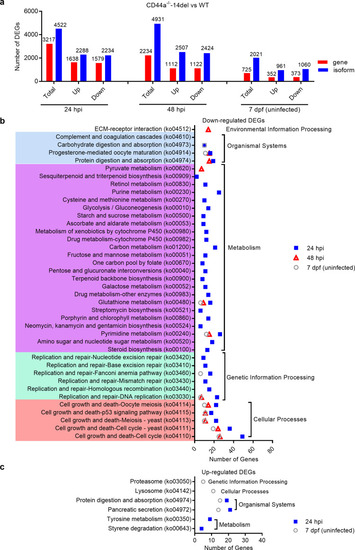
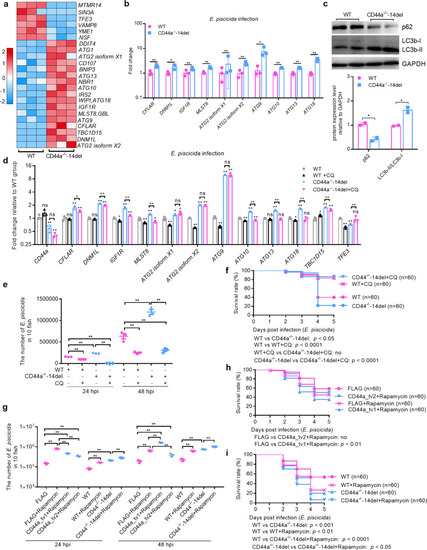
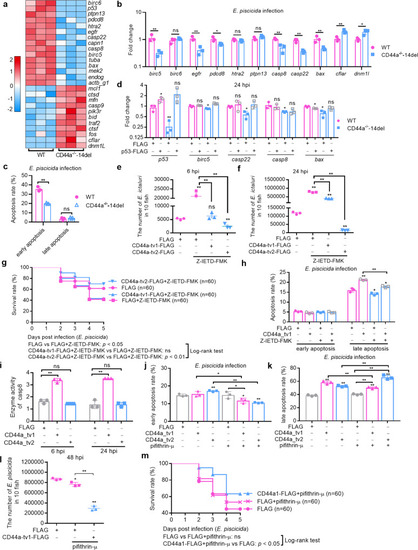
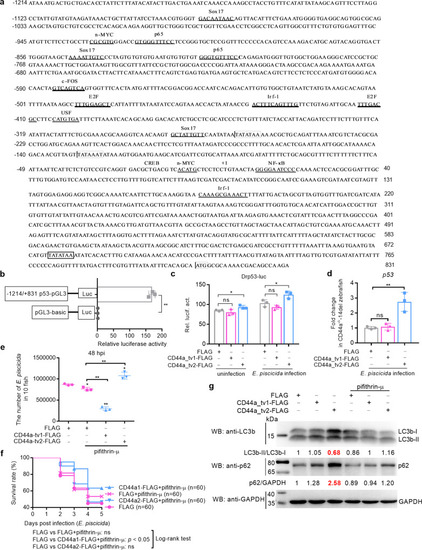
CD44a deficiency regulates the expression levels of many genes involved in p53 signaling, apoptosis and autophagy, which leads to inhibition of apoptosis and induction of autophagy in the case of |