- Title
-
Zebrafish: A Resourceful Vertebrate Model to Investigate Skeletal Disorders
- Authors
- Tonelli, F., Bek, J.W., Besio, R., De Clercq, A., Leoni, L., Salmon, P., Coucke, P.J., Willaert, A., Forlino, A.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
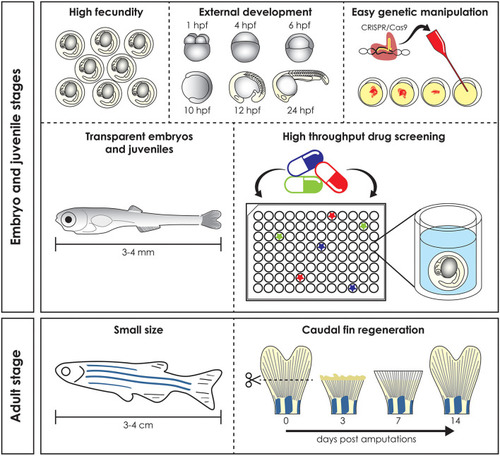
Advantages of the zebrafish model. Zebrafish has several advantages compared to mammal models. High fecundity and external fertilization and development allow easy genomic manipulation, transparent early life stages guarantee |
|
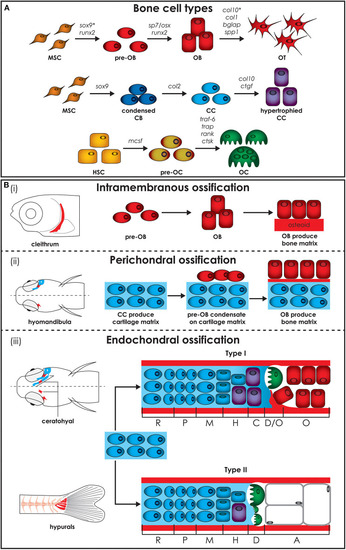
Zebrafish bone cells and ossification types. |
|
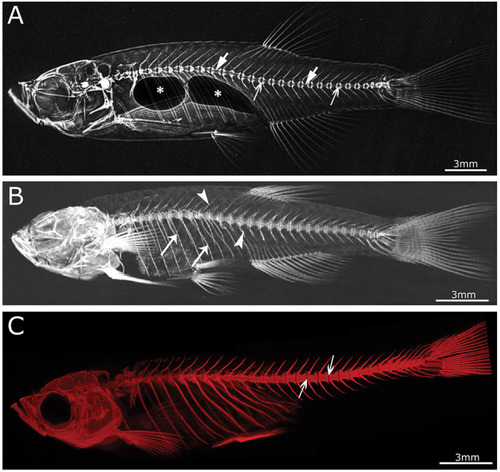
Imaging techniques in zebrafish. |
|
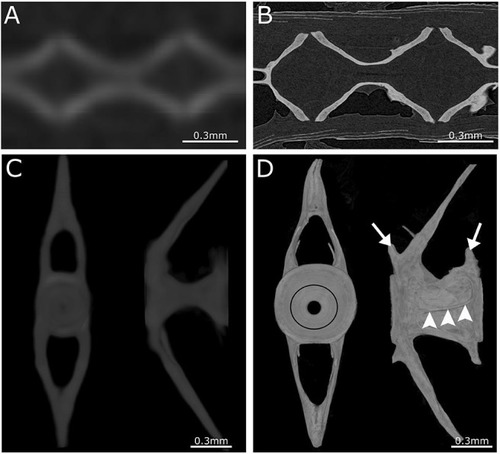
Comparison between low- and high-resolution microCT. |
|
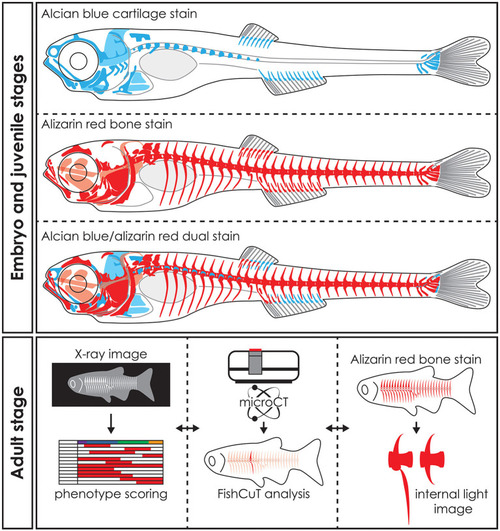
Whole mount staining in early stages and applications of visualization techniques in adult zebrafish. Schematic representation of whole mount cleared and stained early stage zebrafish for cartilage with alcian blue, mineralized tissues (bone) with alizarin red and dual stained for both cartilage and mineralized tissues. Notice that only part of the skull, the basiventrals [for definition see Gadow and Abbott ( |