- Title
-
Pulmonary Exposure to Magnéli Phase Titanium Suboxides Results in Significant Macrophage Abnormalities and Decreased Lung Function
- Authors
- McDaniel, D.K., Ringel-Scaia, V.M., Morrison, H.A., Coutermarsh-Ott, S., Council-Troche, M., Angle, J.W., Perry, J.B., Davis, G., Leng, W., Minarchick, V., Yang, Y., Chen, B., Reece, S.W., Brown, D.A., Cecere, T.E., Brown, J.M., Gowdy, K.M., Hochella, M.F., Allen, I.C.
- Source
- Full text @ Front Immunol
|
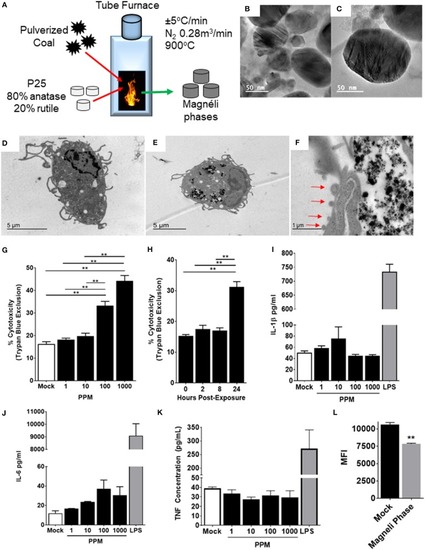
Magnéli phase phagocytosis results in increased cell death in bone marrow-derived macrophages. |
|
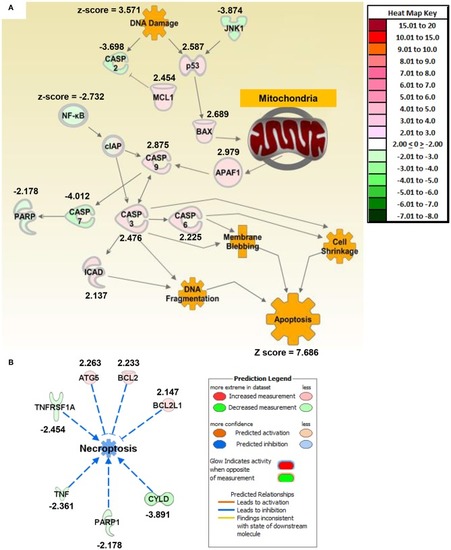
Macrophage exposure to Magnéli phases activates gene expression profiles associated with apoptosis and mitochondria dysfunction. |
|
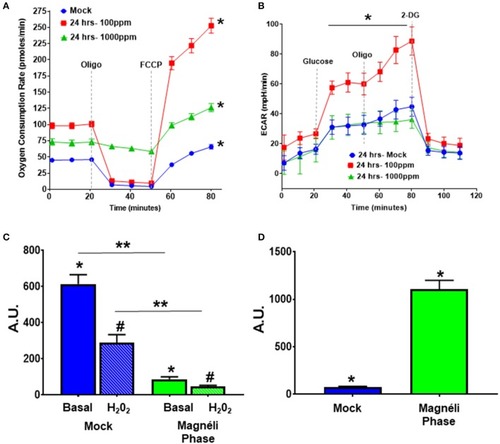
Alterations in cellular energetics and mitochondrial membrane potential in macrophages treated with Ti6O11. |
|
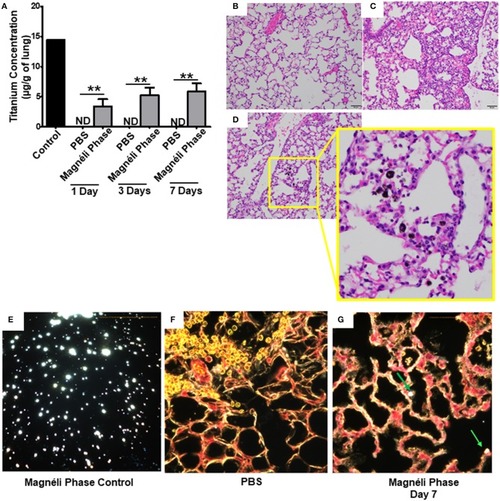
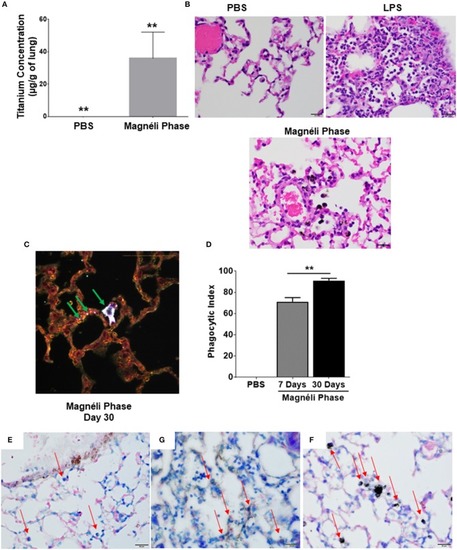
Magnéli phases concentrate in pulmonary macrophages following a single exposure and are retained in the lung. |
|
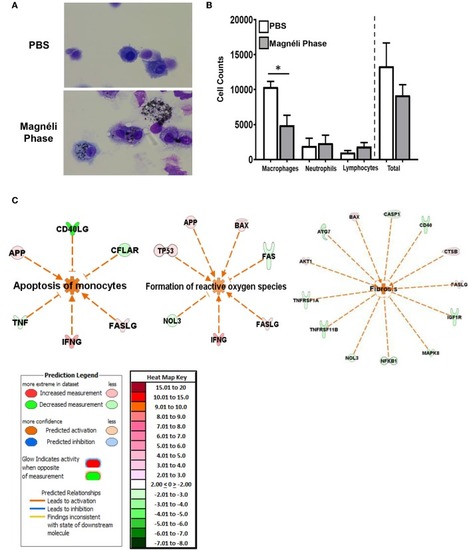
Magnéli phases concentrate in pulmonary macrophages, resulting in significant dysfunction. |
|
Repeated exposures concentrates Magnéli phases in pulmonary macrophages. |
|
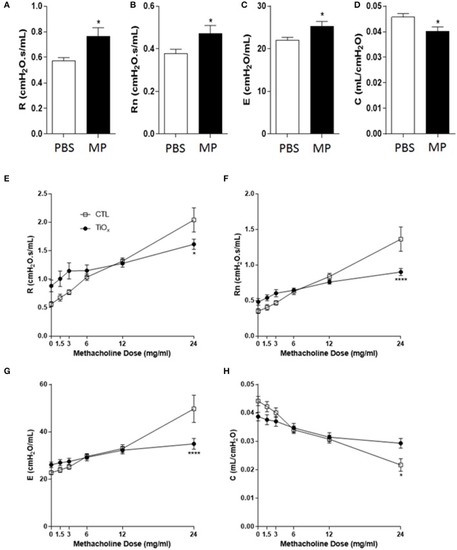
Chronic exposure to Magnéli phases significantly attenuates lung function. |