- Title
-
CiliaCarta: An integrated and validated compendium of ciliary genes
- Authors
- van Dam, T.J.P., Kennedy, J., van der Lee, R., de Vrieze, E., Wunderlich, K.A., Rix, S., Dougherty, G.W., Lambacher, N.J., Li, C., Jensen, V.L., Leroux, M.R., Hjeij, R., Horn, N., Texier, Y., Wissinger, Y., van Reeuwijk, J., Wheway, G., Knapp, B., Scheel, J.F., Franco, B., Mans, D.A., van Wijk, E., Képès, F., Slaats, G.G., Toedt, G., Kremer, H., Omran, H., Szymanska, K., Koutroumpas, K., Ueffing, M., Nguyen, T.T., Letteboer, S.J.F., Oud, M.M., van Beersum, S.E.C., Schmidts, M., Beales, P.L., Lu, Q., Giles, R.H., Szklarczyk, R., Russell, R.B., Gibson, T.J., Johnson, C.A., Blacque, O.E., Wolfrum, U., Boldt, K., Roepman, R., Hernandez-Hernandez, V., Huynen, M.A.
- Source
- Full text @ PLoS One
|
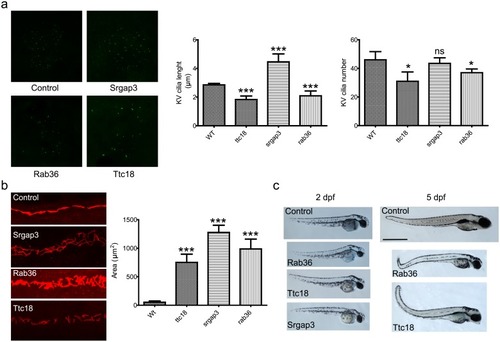
A) Cilia length and number in zebrafish Kupffer’s vesicles. The length and number of cilia in ttc18 and rab36 morphants are significantly reduced (p<0.001 and p<0.05 resp.). Cilia in srgap3 morphants are elongated (p<0.001) but the number of cilia is normal. Bars represent mean ± S.E.M. B) Pronephric ducts in 24 hpf morphants. Cilia are stained with antibodies against acetylated alpha tubulin. The pronephric ducts are significantly enlarged for all three morphants compared to wild type (p<0.001). Bars represent mean ± S.E.M. C) Whole embryo phenotype 2 days post fertilization (dpf) and 5 dpf zebrafish control and morphant embryos. All morphants exhibit the body curvature that is characteristic for cilia dysfunction. Note that in our screening we did not manage to obtain surviving srgap3 morphants past 3 dpf. PHENOTYPE:
|
|
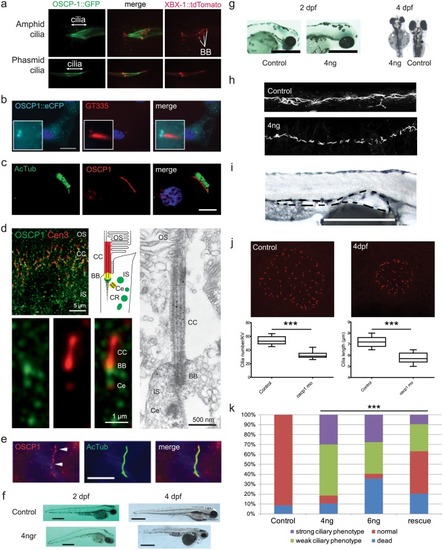
A) GFP-tagged OSCP-1 driven by its endogenous promoter is specifically expressed in ciliated sensory neurons in PHENOTYPE:
|
|
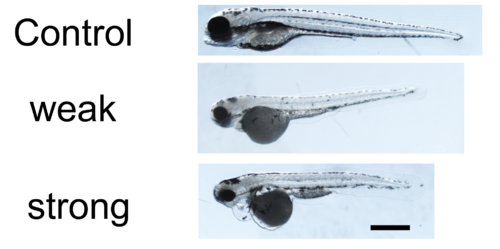
oscp1 zebrafish morpholino phenotypes.Zebrafish strong oscp1 mo phenotype: short body, small eye, obvious pronephric cyst development, and obvious heart edemas. Zebrafish weak oscp1 mo phenotype: short body, small eye, small heads, but no obvious pronephric cyst or heart edemas. |