- Title
-
A familial congenital heart disease with a possible multigenic origin involving a mutation in BMPR1A
- Authors
- Demal, T.J., Heise, M., Reiz, B., Dogra, D., Brænne, I., Reichenspurner, H., Männer, J., Aherrahrou, Z., Schunkert, H., Erdmann, J., Abdelilah-Seyfried, S.
- Source
- Full text @ Sci. Rep.
|
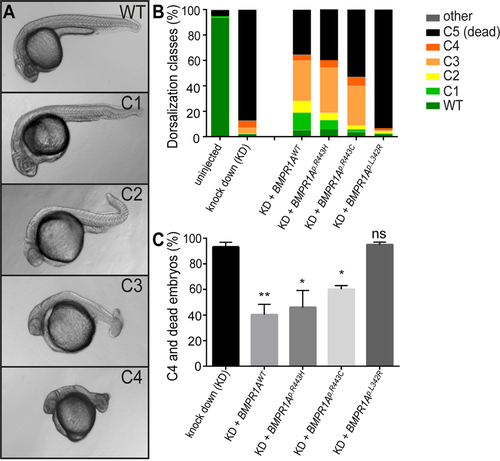
Rescue of bmpr1aa morphant dorsalization phenotypes in zebrafish by injection of mRNA encoding human BMPR1A. (A) Representative classes of dorsalization phenotypes in zebrafish embryos at 24hpf as previously described36. (B) Mean percentages of the five dorsalization classes among the different experimental groups [total number of embryos analysed: uninjected, n = 528; bmpr1aa MO knockdown (KD), n = 217; KD + BMPR1AWT mRNA, n = 228; KD + BMPR1Ap.R443H mRNA, n = 255; KD + BMPR1Ap.R443C mRNA (JPS), n = 147; KD + BMPR1Ap.L342R mRNA (Linkspoot), n = 219]. (C) Share of embryos with C4 phenotype or lethality (mean ± SEM) after knockdown and human BMPR1A mRNA rescue injections by 24hpf [Total number of injected embryos: n = 1066 (Supplementary Tables S6, S7, S8, S9, S10). Number of experiments: KD, n = 5; KD + BMPR1AWT mRNA, n = 6; KD + BMPR1Ap.R443H mRNA, n = 5; KD + BMPR1Ap.R443C mRNA (JPS), n = 4; KD + BMPR1Ap.L342R mRNA (Linkspoot), n = 4. Indicated is the statistical significance of the difference between the occurrence of severe dorzalisation defects in each condition compared with the pure knockdown (KD) (*p’ < 0.05, **p’ < 0.01, ns: not significant)].
PHENOTYPE:
|
|
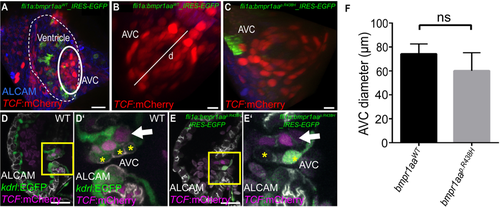
Pan-endothelial expression of a zebrafish Bmpr1aap.R438H mutant protein does not affect embryonic valvulogenesis. (A–E) Shown are reconstructions of confocal z-stack images of the zebrafish embryonic atrioventricular canal (AVC) region. (A) Embryonic cardiac ventricle in a Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aaWT_IRES_EGFP)md65; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5 zebrafish at 120hpf. The AVC region is outlined. Scale bar = 20 µm. (B) AVC region in a Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aaWT_IRES_EGFP)md65; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5 or (C) Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aap.R438H_IRES_EGFP)md60; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5 zebrafish at 120hpf. Indicated is the diameter of the AVC (d). Scale bar = 10 µm. (D,D′) Embryonic valve formation in the WT zebrafish embryo at 72hpf. Box indicates the region shown in (D′). Valve leaflets in WT embryos are characterized by double-layering with an abluminal population of AVC cells that has active Wnt signalling marked by Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5 (arrow). Cell membranes of luminal cells are marked by immuno-labelling against ALCAM (cells marked by asterisks). (E,E′) Similarly, valvulogenesis is not affected in Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aap.R438H_IRES_EGFP)md60; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5 embryos that show a normal double-layered leaflet morphology with Wnt signalling in abluminal cells (arrow) and ALCAM-positive luminal cells (asterisks). Scale bars = 20 µm. (F) The diameter of the AVC in zebrafish embryos with pan-endothelial overexpression of bmpr1aap.R438H does not significantly differ from that upon bmpr1aaWT overexpression [total number of embryos analysed: bmpr1aaWT, n = 6; bmpr1aap.R438H, n = 8; two-sided student’s t-test, p = 0,066].
EXPRESSION / LABELING:
PHENOTYPE:
|
|
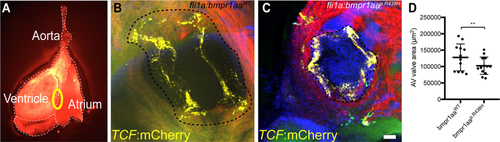
Adult zebrafish with pan-endothelial expression of Bmpr1aap.R438H have smaller AV valves. (A) Fluorescence microscopy image of an adult zebrafish heart (ventral view). Circle indicates the position of the AV valve. (B,C) Confocal z-stack maximum intensity projection of adult AV valves of zebrafish with (B) Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aaWT_IRES_EGFP)md65; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5 or (C) Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aap.R438H_IRES_EGFP)md60; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5. The interrupted line indicates the AV valve annulus. This valve annulus surrounds the edge of the valve leaflets marked by TCF expression and occurs in the confocal images as a black ring. The area bounded by the AV valve annulus was measured to quantify the valve size. The AV valve measurement technique is further explained in Supplementary Fig. S4. Scale bar = 50 µm. (D)Scatter plot of the AV valve area measurements. Adult zebrafish overexpressing Bmpr1aap.R438H show a significant reduction in AV valve area [total number of embryos analysed: bmpr1aaWT, n = 12; bmpr1aap.R438H, n = 13; ANCOVA, F(1, 22) = 10.73; **p = 0.003].
|
|
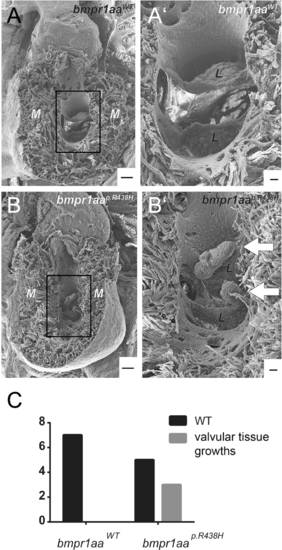
Ectopic valvular tissue growths occur in adult zebrafish overexpressing the bmpr1aap.R438H variant within endocardium. (A,B) Electron microscopic images of adult zebrafish hearts with (A) Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aaWT_IRES_EGFP)md65; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5 or (B) Tg(fli1a:Gal4FF)ubs3; Tg(UAS:bmpr1aap.R438H_IRES_EGFP)md60; Tg(7xTCF-Xia.Sia:NLS-mCherry)ia5. M = myocardium. Scale bar = 100 µm. (A′,B′) Magnified view of the atrioventricular valve. L = valve leaflet. Scale bar = 20 µm. (A) Adult zebrafish hearts with endothelial overexpression of bmpr1aaWT do not have any obvious morphological changes. (B′) 3 out of 8 analysed adult zebrafish hearts overexpressing bmpr1aap.R438H have ectopic valvular tissue growth at the atrioventricular valve (arrows). (C) Numbers of zebrafish hearts overexpressing either bmpr1aap.R438H or bmpr1aaWT with ectopic valvular tissue growth. None out of 7 analysed zebrafish hearts overexpressing bmpr1aaWT had ectopic valvular tissue growths (p = 0.200, fisher’s exact test). PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |