- Title
-
Glycine and Folate Ameliorate Models of Congenital Sideroblastic Anemia
- Authors
- Fernández-Murray, J.P., Prykhozhij, S.V., Dufay, J.N., Steele, S.L., Gaston, D., Nasrallah, G.K., Coombs, A.J., Liwski, R.S., Fernandez, C.V., Berman, J.N., McMaster, C.R.
- Source
- Full text @ PLoS Genet.
|
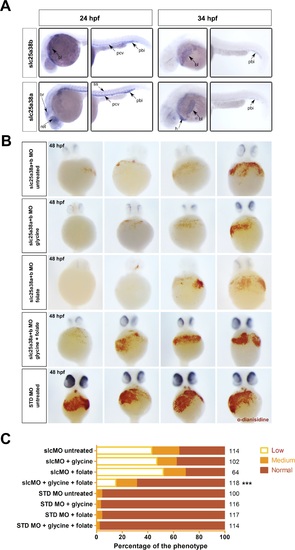
Rescue of the zebrafish model of congenital sideroblastic anemia.A) Whole-mount in situ hybridizations with probes for slc25a38a and slc25a38b at 24 and 34 hpf stages of development demonstrate predominant erythroid expression. For each stage and probe, head and tail views are shown and sites of expression are labeled. Abbreviations: pbi-posterior blood island, pcv-posterior cardinal vein, ss-somites, bl-blood, ret-retina, br-brain, h-heart. Expression of slc25a38b was observed in the posterior blood island, blood and posterior cardinal vein consistent with its preferential expression in erythroid progenitors and erythrocytes, whereas slc25a38a had the same blood-related expression pattern features and was also expressed in retina, brain and somites at 24 hpf. B) Representative images for hemoglobin staining using o-dianisidine of 48 hpf zebrafish embryos injected with slc25a38a+b morpholinos, or standard control morpholino (STD MO), treated with 100 mM glycine, 1 mM sodium folate, or both, starting from 4 hours post-injection. To control for morpholino specificity, we also injected 5-mismatch (5MM) versions of the same slc25a38a+b morpholinos and stained the injected embryos with o-dianisidine. For STD MO and 5MM morphants, images from only the STD MO untreated group are presented because of the low variation in o-dianisidine scores of untreated embryos versus those where glycine and/or folate were present. C) Graph of the o-dianisidine staining heme scores of 48 hpf zebrafish embryos injected with either slc25a38a+b morpholinos (slcMO) or STD MO and then treated from 4 through to 48 hpf with glycine, folate, or both glycine plus folate. All embryos were scored as having “low”, “medium” or “normal” hemoglobin levels in a blinded manner based on visual inspection of o-dianisidine staining. The total numbers (right axis) of scored embryos are indicated, from a total of three independent experiments. The “***” indicates that the p-value between the numbers of embryos in different scoring categories for slc25a38a+b morphants treated with glycine and folate versus the untreated slc25a38a+b morphant group is <0.001. |
|
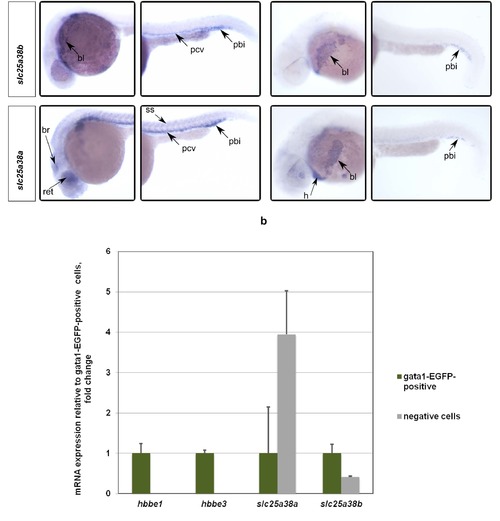
Whole-mount in situ hybridizations with probes for slc25a38a and slc25a38b at 24 and 34 hpf stages of development demonstrate predominant erythroid expression. To confirm preferential expression in red blood cells, EGFP-positive erythrocytes were isolated by FACS from gata1:EGFP (where erythroid lineage cells are labeled by EGFP) transgenic zebrafish embryos. Quantitative PCR assays for hbbe1, hbbe3, slc25a38a and slc25a38b were performed on cDNA made from FACS-sorted positive and negative cells. Gene expression qPCR values were normalized to 18S ribosomal RNA values. Relative expression values to EGFP-positive cells are presented. Error bars indicate standard errors from quantitative PCR experiments done on 3 separate FACS-sorted cell samples. *** p < 0.001, ** p < 0.01, * p < 0.05 for the t-test between expression values for the EGFP-positive and negative cells. EXPRESSION / LABELING:
|
|
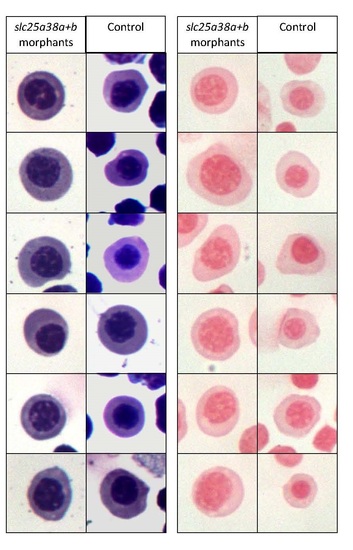
Erythroid cells isolated from slc25a38a+b morphants are morphologically distinct but do not contain any pathological iron deposits compared to cells from control morphants.GFP positive cells were isolated by fluorescence activated cell sorting (FACS) from 48 hpf gata1:EGFP embryos, concentrated onto slides using standard cytospin protocols, and stained with Wright-Giemsa staining for gross morphology and erythroid cell identification (left) and Perls’ Prussian Blue for iron deposits (right). While no pathological iron deposits were detected in the slc25a38a+b morphant cells, these cells are larger with less compact nuclei, indicating a different level of maturation compared with control cells. Images are of randomly selected cells from the same experiment. The experiment was replicated three times with similar results. PHENOTYPE:
|
|
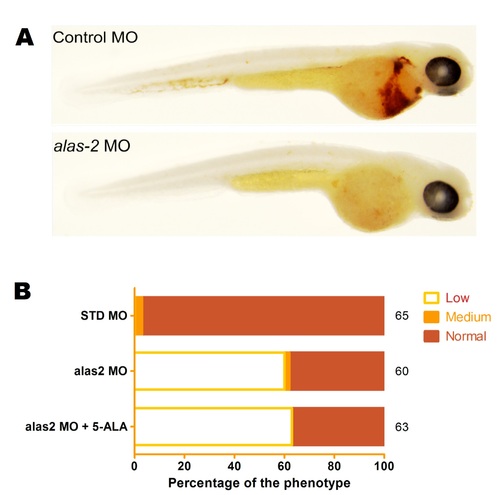
5-Aminolevulinic acid does not restore hemoglobin levels to alas2 or slc25a38a+b morphants.Zebrafish embryos at 48-hpf were stained for hemoglobin with o-dianisidine following microinjection (at 1-cell stage) with the alas2 or control standard MO. A) The typical phenotypes of control standard morpholino (STD MO) and alas2 morpholino-injected embryos stained with o-dianisidine are shown. B) The morphants were incubated with or without 0.3 mM of 5-Ala added to the egg water at 4 hours post-injection. The level of o-dianisidine hemoglobin staining was scored visually as “low”, “medium” or “normal” in a blinded manner and the proportions of embryos in each category are presented in the graph. Results of three separate experiments are presented with the actual numbers of scored embryos indicated on the right hand axis of the graph. There was no statistically significant difference between untreated and 5-Ala-treated morphants indicating that 5-Ala supplementation did not increase hemoglobin level. PHENOTYPE:
|