- Title
-
An early requirement for nkx2.5 Ensures first and Second heart field ventricular identity and cardiac function into adulthood
- Authors
- George, V., Colombo, S., Targoff, K.L.
- Source
- Full text @ Dev. Biol.
|
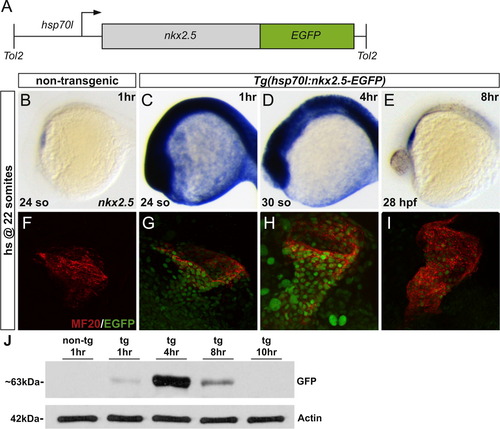
Heat-shock inducible transgene, Tg(hsp70l:nkx2.5-EGFP), allows for temporally controlled expression of nkx2.5. (A) Schematic representation of the heat-shock inducible transgene. (B-E) In situ hybridization depicts expression of nkx2.5 in non-transgenic (B) and Tg(hsp70l:nkx2.5-EGFP) (C-E) embryos. Lateral views, anterior to the left. Following initiation of heat shock (hs) at 22 somites (20 hpf), non-transgenic embryos demonstrate endogenous nkx2.5 expression in the heart tube at 24 somites (1 h post-hs) (B). In comparison, heat-shocked transgenic embryos reveal upregulation of nkx2.5 ubiquitously in the embryo at 24 somites (1 h post-hs) (C) and at 30 somites (4 h post-hs) (D). By 28 hpf (8 h post-hs), global nkx2.5 expression is significantly diminished and only cardiac specific expression remains (E). Time interval post-hs is indicated in the upper right corner of each panel. (F–I) MF20 immunofluorescence (red) indicates cardiac myosin heavy chain in all cardiomyocytes and EGFP (green) reflects transgenic expression following initiation of heat shock at 22 somites (20 hpf). Lateral views, anterior to the right. Confocal projections of fixed, dissected non-transgenic (F) and transgenic (G–I) hearts. EGFP can be visualized as early as 24 somites (1 h post-hs) (G) with strong perdurance through 30 somites (4 h post-hs) (H). Yet, only minimal residual expression is evident by 28 hpf (8 h post-hs) (I). (J) Western blots using anti-GFP and anti-Actin on protein extracts prepared from non-transgenic and transgenic embryos following heat shock. Samples were collected at 1 h, 4 h, 8 h, and 10 h post-hs, respectively. The full-length Nkx2.5-EGFP runs with apparent molecular weight of ~63 kDa. The same membrane was probed with anti-Actin, serving as a loading control. EXPRESSION / LABELING:
|
|
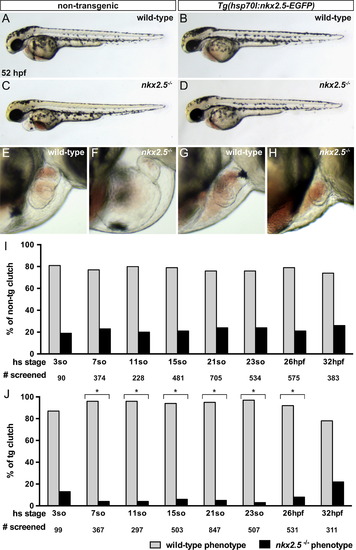
Early overexpression of nkx2.5 rescues late morphological defects in nkx2.5-/- embryos. (A–D) Lateral views of live embryos, anterior to the left, at 52 hpf. All embryos were heat-shocked at 21 somites. Aside from cardiac defects and pericardial edema, the non- transgenic nkx2.5-/- embryos appear morphologically normal (C). While heat shock of wild-type embryos yields no evidence of toxicity (B), the cardiac defects and pericardial edema in the nkx2.5-/-;Tg(hsp70l:nkx2.5-EGFP) embryos are dramatically improved (D). (E–H) Lateral views of live embryos, anterior to the right, at 52 hpf. Compared to non-transgenic (E) and transgenic (G) wild-type hearts, the non-transgenic nkx2.5-/- heart (F) is unlooped and has striking defects in both ventricular and atrial morphologies. In contrast, the transgenic nkx2.5-/- heart (H) resembles its wild-type sibling (G) indicating a rescue of chamber proportions. (I,J) Bar graphs illustrate the percentage of phenotypically wild-type (gray) and nkx2.5-/- (black) embryos in multiple clutches of non-transgenic (I) and transgenic (J) siblings following heat shock at specific developmental stages. Gross phenotypic assessment was performed assaying for morphological features noted in (A-H) at 52 hpf. The total number of embryos screened at each time point is noted. Fisher exact tests (one-sided; p<0.01) were performed; asterisks indicate statistically significant differences between proportions of non-transgenic (I) and transgenic (J) wild-type and phenotypically nkx2.5-/- embryos. A notable decrement in the percentage of transgenic nkx2.5-/- embryos between 7 somites and 26 hpf denotes rescue of morphological ventricular and atrial defects. PHENOTYPE:
|
|
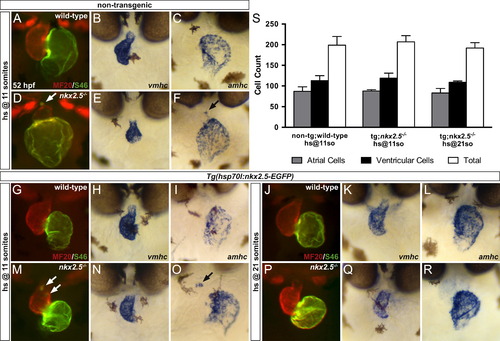
Early expression of nkx2.5 actively maintains ventricular identity during chamber emergence. (A-R) Ventral views, anterior to the top, at 52 hpf in non-transgenic (A-F) and Tg(hsp70l:nkx2.5-EGFP) (G-R) embryos. MF20/S46 immunofluorescence (A,D,G,J,M,P) distinguishes ventricular myocardium (red) from atrial myocardium (green) and in situ hybridization depicts expression of vmhc (B,E,H,K,N,Q) and amhc (C,F,I,L,O,R) in genotypically wild-type (A-C,G-I,J-L) and nkx2.5-/- (D-F,M-O,P-R) embryos. In comparison to non-transgenic nkx2.5-/- embryos (D-F), transgenic nkx2.5-/- embryos retain appropriate chamber-specific identity following heat shock at 11 somites (M-O) and 21 somites (P-R). However, ectopic S46+ cardiomyocytes near the OFT in the non-transgenic nkx2.5-/- embryo (D) are also present in the transgenic nkx2.5-/- embryo following heat shock at 11 somites (M), whereas complete rescue of ventricular chamber identity is achieved following heat shock at 21 somites (P). White arrows indicate ectopic S46+ cells (D,M). Black arrows highlight the corresponding ectopic amhc+ population (F,O). (S) Bar graph indicates numbers of atrial, ventricular, and total cardiomyocyte nuclei; the transgene Tg(-5.1myl7:nDsRed2) in both cardiac chambers facilitates cell counting at 48 hpf. Mean and standard error of each data set are shown without detection of statistically significant differences between non-transgenic wild-type and transgenic nkx2.5-/- embryos following heat shock at 11 somites and 21 somites (p>0.01), indicating rescue of chamber-specific cardiomyocyte cell numbers in transgenic nkx2.5-/- siblings. All embryos were genotyped following cardiomyocyte cell counting. |
|
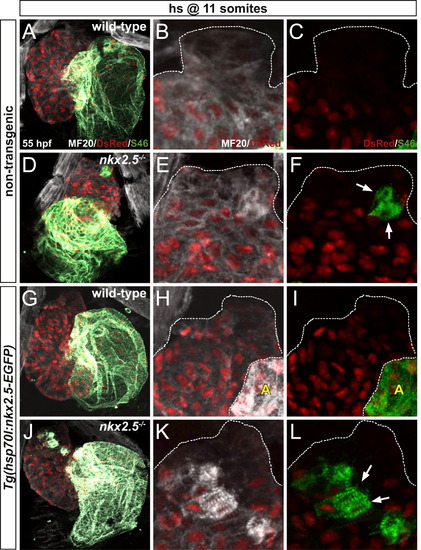
nkx2.5 is necessary to maintain ventricular identity in late-differentiating cardiomyocytes derived from the SHF. MF20/S46 immunofluorescence distinguishes ventricular myocardium (white) from atrial myocardium (green) in non-transgenic (A-F) and Tg(hsp70l:nkx2.5-EGFP) (G-L) embryos. Confocal projections of wild-type (A-C, G-I) and nkx2.5-/- (D–F, J–L) hearts depict cardiomyocyte nuclei with Tg(-5.1myl7:nDsRed2) (red). Ventral views, arterial pole to the top, at 55 hpf. (B,C,E,F,H,I,K,L) White dots outline the MF20+ cardiomyocyte borders in the OFTs of hearts in (A,D,G,J), respectively. White arrows indicate ectopic S46+ cells; “A” denotes atrium. All embryos were heat-shocked at 11 somites. (A–C) In wild-type non-transgenic hearts, the late-differentiating cardiomyocyte population exhibits MF20, but not DsRed, fluorescence due to the delay in expression of Tg(-5.1myl7:nDsRed2) at the arterial pole. (D–F) Similarly, in non-transgenic nkx2.5-/- hearts, cardiomyocytes expressing MF20, but not DsRed, are present at the arterial pole. A few ectopic S46+ cardiomyocytes are also visualized in this region (DsRed nuclei). (G–I) In transgenic wild-type hearts, MF20, but not DsRed, fluorescence at the arterial pole designates the delayed differentiation of these SHF-derived cardiomyocytes. (J–L) Despite the rescue of the cardiac chamber morphology and identity, excess S46+ cardiomyocytes are visualized at the arterial pole of transgenic nkx2.5-/- hearts where the late-differentiating population accretes (DsRed nuclei). EXPRESSION / LABELING:
|
|
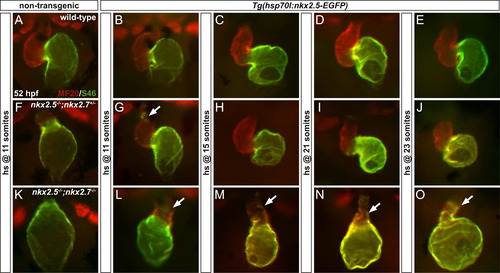
Resupplying nkx2.5 in nkx2.5/;nkx2.7/ embryos reveals dose-dependent functions of nkx genes. Frontal views, anterior to the top, of MF20/S46 immunofluorescence (as in Fig. 3) at 52 hpf. In non-transgenic wild-type (A) and Tg(hsp70l:nkx2.5-EGFP) embryos (B-E), cardiac morphology and chamber-specific identity are maintained following heat shock at 11 somites through 23 somites. In contrast, non-transgenic nkx2.5-/-;nkx2.7+/- (F) and nkx2.5-/-;nkx2.7-/- (K) embryos have enlarged atrial chambers, underdeveloped or indiscernible ventricular chambers, and diminished outflow tracts. Following heat shock at the same time points as performed in the wild-type embryos, transgenic nkx2.5-/-;nkx2.7+/- embryos (G-J) exhibit substantial improvement in ventricular chamber size with only a few residual ectopic S46+ cardiomyocytes remaining in embryos treated at 11 somites (G). However, only moderate rescue of abnormalities in morphology and identity is achieved in the transgenic nkx2.5-/-;nkx2.7-/- embryos following heat shock at 11 somites and 15 somites (L,M) and only minimal rescue is achieved following heat shock at 21 somites and 23 somites (N,O). White arrows indicate ectopic S46+ cardiomyocytes. |
|
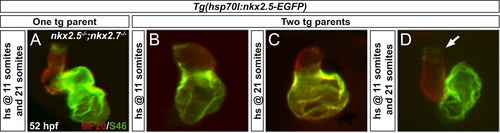
Rescue of nkx2.5-/-;nkx2.7-/- embryos validates a dose-dependent role of nkx2.5 and suggests a unique function for nkx2.7. Frontal views, anterior to the top, of MF20/S46 immunofluorescence (as in Fig. 3) at 52 hpf. In contrast to nkx2.5-/-;nkx2.7-/- offspring of a single Tg(hsp70l:nkx2.5-EGFP) carrier following heat shock at one time point (Fig. 5L-O), a transgenic nkx2.5-/-;nkx2.7-/-embryo subject to heat at 11 somites and 21 somites demonstrates enhanced rescue of ventricular and atrial size and identity defects (A). Despite only moderate improvement in offspring from a cross of two transgenic parents at 11 somites (B) and 21 somites (C), performing heat shock at two time points to augment Tg(hsp70l:nkx2.5-EGFP) expression further yields normalization of cardiac chamber morphology (D). Yet, residual, ectopic S46+ cardiomyocytes highlight chamber identity abnormalities in the late-differentiating SHF-derived population (D; arrow). EXPRESSION / LABELING:
|
|
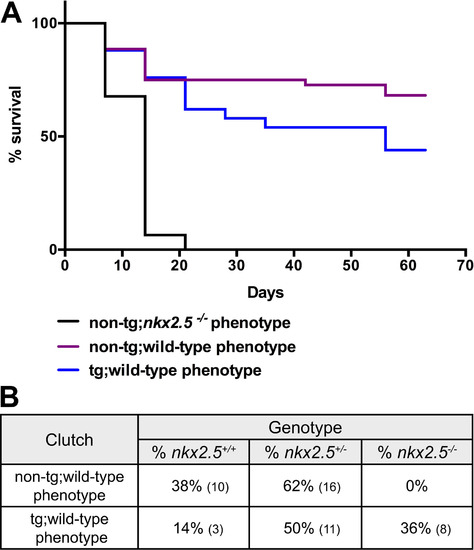
Re-expression of nkx2.5 prior to heart tube elongation results in healthy nkx2.5-/- adults. (A) Kaplan-Meier survival curve depicts the pattern of larval death following phenotypic assessment of non-transgenic nkx2.5-/- embryos in addition to non-transgenic and Tg(hsp70l:nkx2.5-EGFP) wild-type embryos. All animals were exposed to heat shock at 21 somites, sorted according to EGFP fluorescence, and subsequently screened morphologically at 52 hpf. While non-transgenic nkx2.5-/- embryos perish within the first 3 weeks, 65% and 44% survival is observed in the non-transgenic and transgenic wild-type clutches, respectively. (B) Genotyping results for the non-transgenic phenotypically wild-type cohort and transgenic phenotypically wild-type cohort reveal nkx2.5-/- embryos in the transgenic rescued group only. Percentages represent the proportion of embryos with a particular genotype in each phenotypic cohort; the number of embryos in each group is noted in parentheses. PHENOTYPE:
|
|
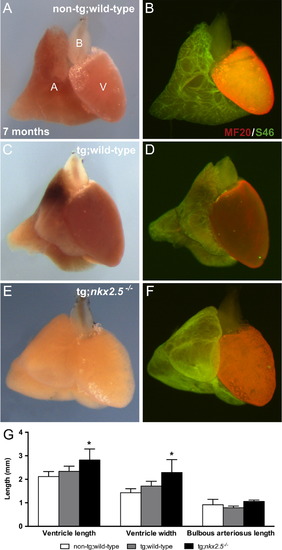
Adult rescued nkx2.5-/- fish exhibit normal cardiac morphology and chamber identity. Whole mount images of 7-month-old non-transgenic (A,B) and Tg(hsp70l:nkx2.5-EGFP) (C-F) wild-type (A-D) and nkx2.5-/- (E,F) hearts. MF20/S46 immunofluorescence distinguishes ventricular (red) from atrial (green) myocardium. Dissected hearts are positioned ventrally with arterial poles to the top. “A” denotes atrium, “V” denotes ventricle, and “B” denotes bulbous arteriosus. All embryos were originally heat-shocked at 21 somites. All fish were genotyped prior to dissection for morphometric analyses. (A,C,E) Similar cardiac morphology is depicted in non-transgenic and transgenic wild-type and transgenic rescued adult nkx2.5-/- hearts. (B,D,F) Cardiac chamber identity is maintained following heat shock at 21 somites in non-transgenic and transgenic wild-type and transgenic rescued adult nkx2.5-/- hearts. (G) Bar graph indicates ventricle length (VL), ventricle width (VW) and bulbous arteriosus length (BAL) in non-transgenic wild-type (n=8), transgenic wild-type (n=7), and transgenic nkx2.5/ (n=2) fish. Mean and standard error of each data set are shown with detection of statistically significant differences between VL and VW of transgenic rescued nkx2.5-/- and non-transgenic wild-type fish (p<0.01). |
Reprinted from Developmental Biology, 400(1), George, V., Colombo, S., Targoff, K.L., An early requirement for nkx2.5 Ensures first and Second heart field ventricular identity and cardiac function into adulthood, 10-22, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.