- Title
-
Growth differentiation factor 6 as a putative risk factor in neuromuscular degeneration
- Authors
- DuVal, M.G., Gilbert, M.J., Watson, D.E., Zerulla, T.C., Tierney, K.B., Allison, W.T.
- Source
- Full text @ PLoS One
|
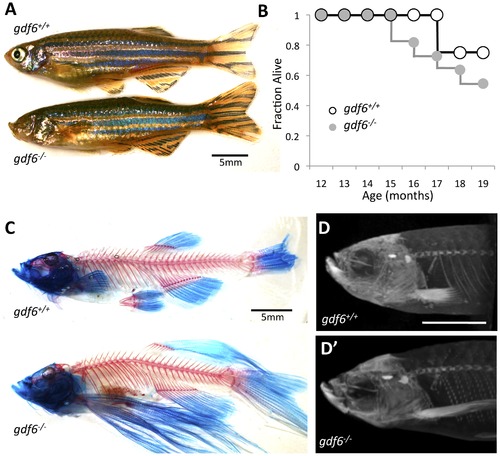
Zebrafish harboring homozygous mutations in gdf6 can be raised to adulthood and do not display overt skeletal defects. A. gdf6-/- fish are viable into adulthood, exhibit variably penetrant microphthalmia and normal body morphology. B. gdf6-/- fish exhibit somewhat decreased survival compared to gdf6+/+ siblings (n = 11 gdf6-/- fish; n = 4 gdf6+/+ siblings). C, D. gdf6-/- fish lack overt skeletal phenotypes, as revealed by (C) clearing and staining or by (D) microCT analysis. The latter is further represented as Supplemental Movie S1. Scale bars are 5 mm. A variety of fin morphologies were present in the fish examined, but these were neither different between experimental groups (genotypes) nor a significant covariant with swim performance (see Results). PHENOTYPE:
|
|
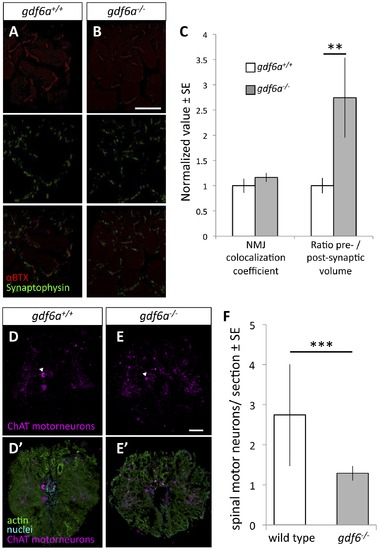
Zebrafish harboring homozygous mutations in gdf6 exhibit altered neuromuscular junctions and fewer spinal motor neurons. A-B. Assessment of neuromuscular junction (NMJ) morphology including presynaptic (synaptophysin) and postsynaptic (αBTX) compartments in 9 month old gdf6-/- and gdf6+/+ siblings by immunohistochemistry. C. ALS-like increases in motoneuron pre-synaptic volumes are observed in gdf6-/- fish when normalized to post-synaptic volumes (**p = 0.009, n = 5 fish per genotype). Coefficients of colocalization for pre- and post-synaptic compartments are not altered, as is expected in later-stage zebrafish ALS models. D-E. Motor neuron cell bodies were identified in cross-sections of spinal cord using immunohistochemistry against choline acetyl transferase (ChAT, e.g. arrowheads) in nine month old gdf6+/+ and gdf6-/- fish (panels A and B, respectively). Bottom panels affirm motoneuron cell body identification using actin and nuclear counter-stains. F. gdf6-/- fish have approximately 50% the abundance of spinal motor neurons compared to sibling gdf6+/+ fish. (Mann-Whitney U Test, *** p<0.001, n>50 sections from 4 fish per genotype, researcher blinded to genotype during quantification). Scale bar = 60 μm in A,B and 50 μm in D,E. |
|
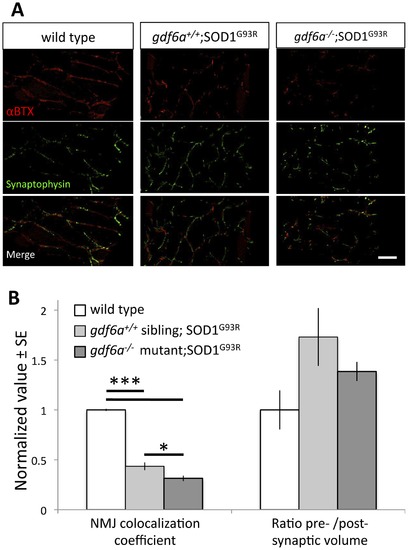
Disruption of gdf6 function exacerbates neuromuscular junction abnormalities in ALS model zebrafish. ALS model zebrafish possess disruptions to neuromuscular junctions (NMJ), and loss of gdf6 function exacerbates this by 7 months of age. A. The presynaptic junctions (labeled with synaptophysin antibody) and postsynaptic junctions (labeled with fluorescently tagged αBTX) in ALS model zebrafish expressing the mutant SOD1G93R show punctate morphology, deviations in presynaptic volume and less overall colocalization compared to WT sibling junctions. Some abnormalities are exacerbated in bigenic siblings expressing the mutant SOD1G93R that are also gdf6-/- (scale bar is 40 µm). B. Quantification of these NMJs suggests the presynaptic/postsynaptic volume ratios of SOD1G93R and bigenic gdf6-/-;SOD1G93R zebrafish are larger than those of WT siblings at this age, though these differences do not rise to statistical significance (Kruskall-Wallis ANOVA, p = 0.134; n = 6,4,6 for WT, SOD1G93R, and bigenic fish respectively). Colocalization coefficients, that measure overall colocalization of presynaptic and postsynaptic junctions, are altered in these fish. The values for SOD1G93R zebrafish are significantly lower than wild type sibling values, indicating that presynapses and postsynapses overlap less, as characterized previously for this transgenic ALS model [28]. Bigenic SOD1G93R zebrafish that are also gdf6-/- have a dramatically lower coefficient than either sets of siblings, including being 30% lower than ALS model SOD1G93R fish with normal gdf6a (*p<0.05; ***p<0.001. Kruskall-Wallis ANOVA with pairwise comparisons). |
|
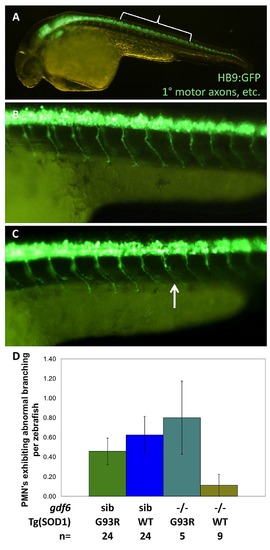
In larval zebrafish, mutations in gdf6a do not appreciably sensitize SOD1ΛG93R zebrafish to develop ALS-like symptoms. Four genotypes combining Gdf6-/- alleles and SOD1ΛG93R alleles were examined in Tg(HB9:eGFP) zebrafish expressing GFP in the axons of primary motor neurons (PMN), or via immunohistochemistry. A. Bracket indicates position of axons quantified, magnified in B,C. B. Normal primary motor axons. C. An example of an abnormal PMN axon (arrow). D. Quantification of primary motor axons in 30 hour post-fertilization embryosshow no difference based on gdf6a genotype; Thus effects are not developmental, and accord with a late-onset phenotype. Results indicated no significant effect of Gdf6 on the presence of axonopathies (p≥0.315, n≥5 larvae per genotype), although the highest rate of PMN axon abnormalities were observed in gdf6a-/-;SOD1+/G93R larvae. EXPRESSION / LABELING:
|