- Title
-
Hematopoietic stem cell development is dependent on blood flow
- Authors
- North, T.E., Goessling, W., Peeters, M., Li, P., Ceol, C., Lord, A.M., Weber, G.J., Harris, J., Cutting, C.C., Huang, P., Dzierzak, E., and Zon, L.I.
- Source
- Full text @ Cell
|
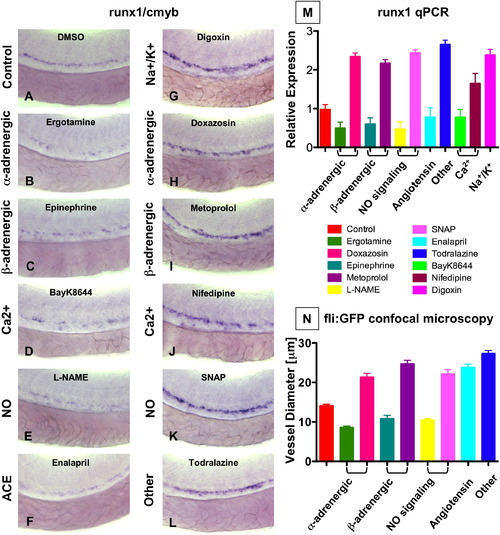
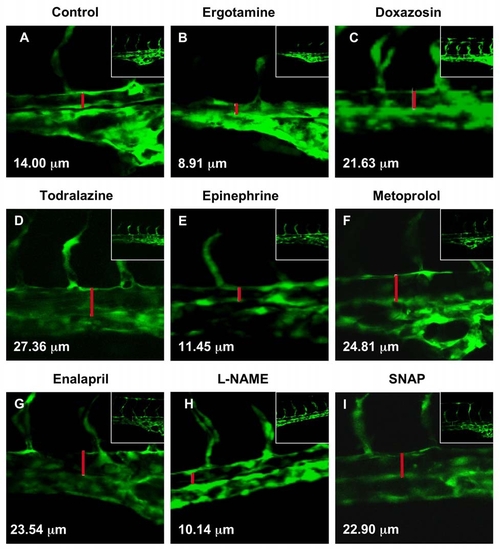
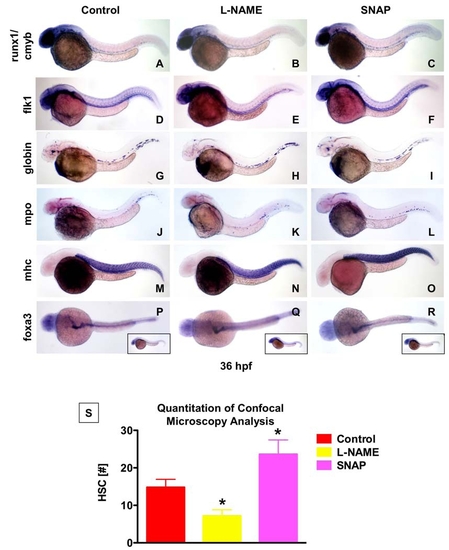
Modulation of Vascular Flow Affects HSC Formation in Zebrafish (A–M) Effect of blood flow modifiers on runx1/cmyb+ HSC formation. Zebrafish were exposed to chemicals (10 μM) from 10 somites–36 hpf and subjected to runx1/cmyb in situ hybridization. Photomicrographs were taken with Nomarski optics at 40x magnification. Representative examples from after drug treatment are shown. (L) Effect of todralazine (10 μM; 67 inc/84). (M) Effect of drug treatment on runx1 expression, quantified by qPCR (mean ± SD). (N) Effect of drug treatment on the diameter of the dorsal aorta in vivo. Transgenic fli:GFP fish were treated with chemicals and imaged by confocal microscopy at 36 hpf; all treatments were statistically significant from the control (mean ± SD, ANOVA, p < 0.001). EXPRESSION / LABELING:
|
|
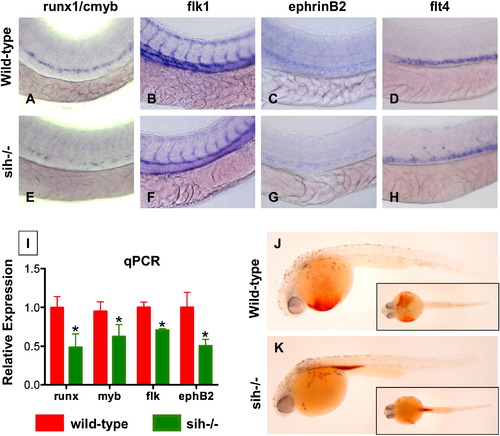
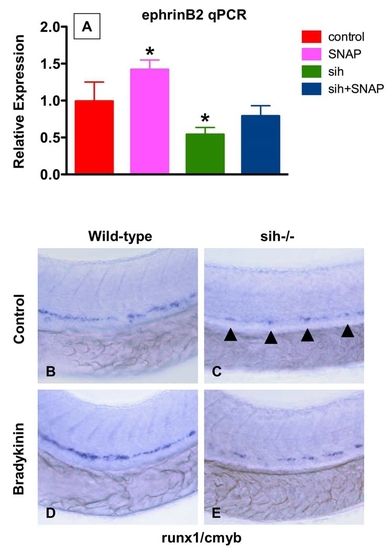
A Beating Heart Is Required for HSC Formation and Artery Development (A–H) Effect of sih mutation on HSC and vascular formation at 36 hpf. (A and E) runx1/cmyb expression is greatly reduced in sih-/- embryos compared to WT siblings. (B and F) flk1 expression reveals a grossly normal vascular pattern in sih-/- embryos; changes in the development of the intersomitic vessels and vascular plexus were noted in some animals. (C and F) ephB2 expression is diminished in sih-/- embryos. (D and H) flt4 expression is expanded in sih-/- embryos. (I) The expression levels of HSC (runx1, cmyb), vascular (flk), and arterial (ephB2) markers are significantly decreased in sih-/- embryos compared to sibling controls (mean ± SD, t test, p < 0.05, n = 3), as measured by qPCR at 36 hpf. (J and K) The sih mutation has no effect on primitive hematopoiesis as seen by benzidine staining at 36 hpf; in the absence of a heartbeat blood is pooled in the major vessels. |
|
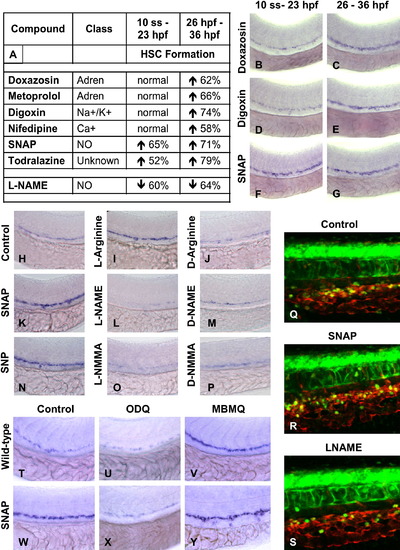
NO Signaling prior to the Onset of Cardiac Activity can Affect HSC Formation (A–G) Effect of vasoactive drugs (10 μM) on HSC formation before and after the onset of heartbeat at 24 hpf, after exposure to chemicals from either 10 somites–23 hpf or from 26–36 hpf. (A) Most vasoactive drugs do not affect HSC formation when applied prior to the onset of heartbeat, while NO modifiers influenced HSC development even prior to heart beat initiation. The percentage of embryos (n > 20) with altered runx1/cmyb expression is indicated. (B–G) Representative examples of flow-modifying drugs on runx1/cmyb expression. (H–P) Specificity of NO signaling in HSC formation. NO donors enhanced and inhibitors diminished HSCs; inactive D-enantiomers had no effect. (Q–S) Effects of NO modulation on HSC number by in vivo confocal imaging in cmyb:GFP; lmo2:dsRed transgenic embryos. (T–Y) Effects of downstream modifiers of NO signaling on runx1/cmyb expression. (U and X) Inhibition of soluble guanyl cyclase by ODQ (10 μM) decreases runx1/cmyb expression in WT and SNAP treated embryos. (V and Y) Inhibition of PDE V by MBMQ (10 μM) increases HSC formation in WT embryos and further enhances the effects of SNAP. EXPRESSION / LABELING:
|
|
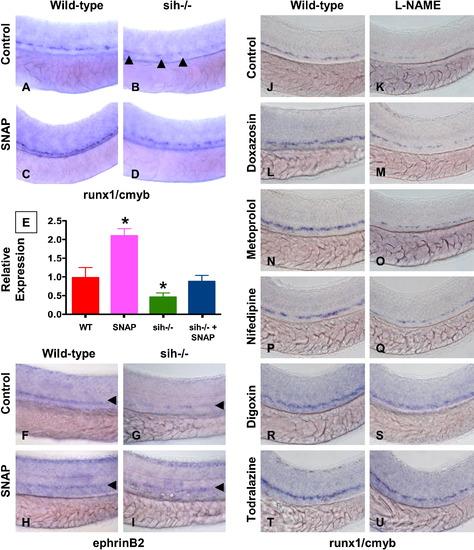
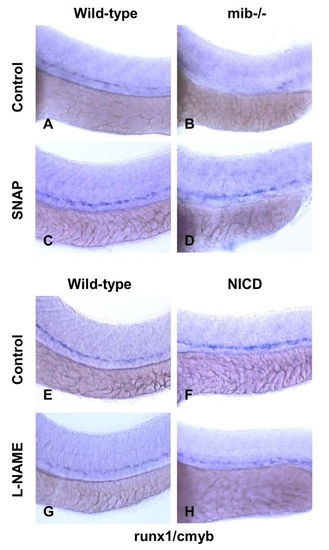
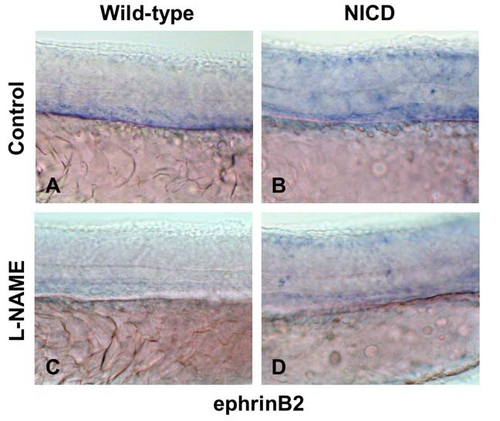
NO Signaling Affects Zebrafish HSC Formation Independent of Heartbeat (A–I) WT and sih-/- mutants were exposed to DMSO and SNAP (10 μM) from 10 somites–36 hpf. (A–D) In situ hybridization for runx1/cmyb. SNAP rescues HSC formation in sih-/- mutants. (B) Remaining runx1/cmyb+ cells are highlighted by arrowheads. (E) qPCR for runx1. * statistically significant versus the WT, mean ± SD, ANOVA, p < 0.001, n = 5. (F–I) Effect of heartbeat and SNAP on ephrinB2 expression, highlighted by arrowheads. (J–U) Effect of L-NAME on HSC formation in embryos concurrently treated with blood flow-modifying agents. L-NAME inhibits the effects of doxazosin ([M], 7 inc/36 observed), metoprolol ([O], 3 inc/31) and nifedipine ([Q], 4 inc/28), but not of digoxin ([S], 16 inc/ 29) and todralazine ([U], 20 inc/33). EXPRESSION / LABELING:
|
|
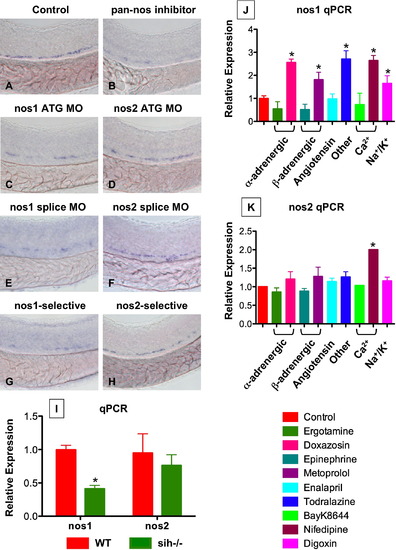
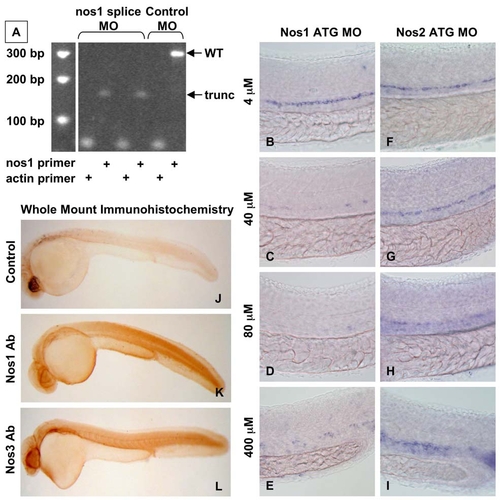
nos1 Is Required for HSC Formation in Zebrafish (A–H) In situ hybridization for runx1/cmyb at 36 hpf. (C and E) nos1 knockdown (40 μM) decreased HSC formation. (D and F) MO (ATG and splice site) against nos2 (40 μM) had no effect on HSC development. (B, G, and H) Chemical nos inhibition confirmed the specific requirement for nos1: embryos exposed to nonspecific (L-NAME; 10 μM) and nos1-selective (S-methyl-L-thiocitrulline; 10 μM) inhibitors demonstrated decreased HSC formation; nos2-selective inhibition (1400W; 10 μM) had minimal impact. (I) WT and sih-/- embryo extracts (n = 20) were subjected to qPCR (mean ± SD; * nos1, WT versus sih, t test, p < 0.001, n = 3; nos2, WT versus sih, p = 0.385, n = 3.). (J and K) Effect of flow-modifying chemicals (10 μM, 10 somites–36 hpf) on nos1 and nos2 expression; nos1 is significantly regulated by most compounds tested. Mean ± SD; * significant versus control, ANOVA, p < 0.01, n = 3. |
|
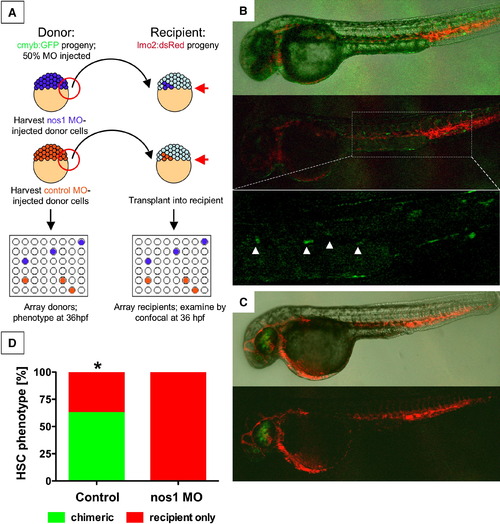
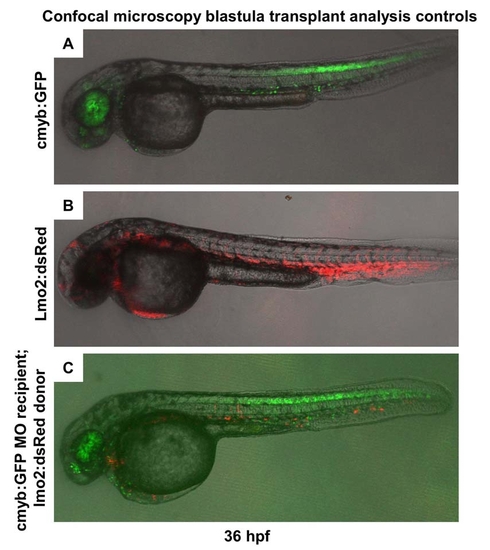
The Effect of NO Signaling on HSC Development Is Cell Autonomous (A) Cells from cmyb:GFP transgenic donor embryos, injected with nos1 ATG MO or control MO, were transplanted into lmo2:dsRed recipients at the blastula stage. (B) Donor contribution to HSC formation assessed by confocal microscopy at 36 hpf. Shown are the merged picture on the top, red/green merge in the middle, and a high-magnification view of green fluorescence only on the bottom. cmyb:GFP donor-derived HSCs in recipients are highlighted by arrowheads. (C) nos1 MO donors never contributed to HSC formation; the presence of cmyb:GFP-derived donor cells in the eye is indicative of a successful transplant. (D) HSC chimerism in transplanted embryos (control versus nos1 MO, Fisher′s exact test, p = 0.0065, n ≥ 8). |

The Effect of NO Signaling on HSC Development in the AGM Is Conserved in Mice (A–H) FACS analysis of dissociated AGM cells in WT and Nos KO mice at e11.5. Nos3-/- mice exhibited a decrease in the Sca1+/cKit+ and CD45+/VE-Cadherin+ populations, while deletion of Nos1 had no significant effects. (I–L) Histological sections through the AGM region of e11.5 embryos; the inset represents a high-magnification view around the hematopoietic clusters. L-NAME exposure causes absence of hematopoietic clusters; Nos3-/- mice exhibit smaller cluster size, while Nos1-/- does not impair cluster formation. Serial sections through the entire aorta of at least ten embryos per genotype/treatment were analyzed. (M and N) Effect of NO signaling on AGM HSC function. AGM regions of somite stage-matched WT, L-NAME treated or Nos3-/- progeny were subdissected at e11.5 and transplanted into sublethally irradiated recipients. L-NAME exposure or Nos3 deletion in embryos significantly reduced CFU-S12 spleen colony formation (mean ± SD; * sig versus control; p < 0.001; ** sig versus L-NAME; p < 0.05; ANOVA, n ≥ 5) (M). Diminished NO signaling significantly decreased embryonic donor cell chimerism rates in individual recipient mice at 6 weeks after transplant (* sig versus control, p < 0.05, ANOVA, n ≥ 5) (N). |
|
Nitric Oxide mediates the effect of blood flow on HSCs. |
|
The silent heart mutation does not affect primitive hematopoiesis or mesodermal and endodermal development at 36 hpf. |
|
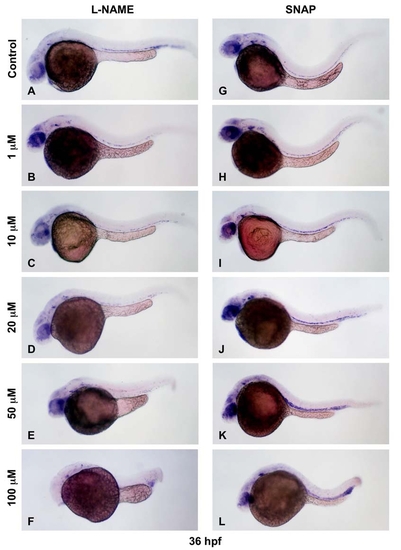
Modulation of NO has dose-dependent effects on HSC formation. |
|
NO modulation does not affect primitive hematopoiesis or development of mesodermal and endodermal structures. |
|
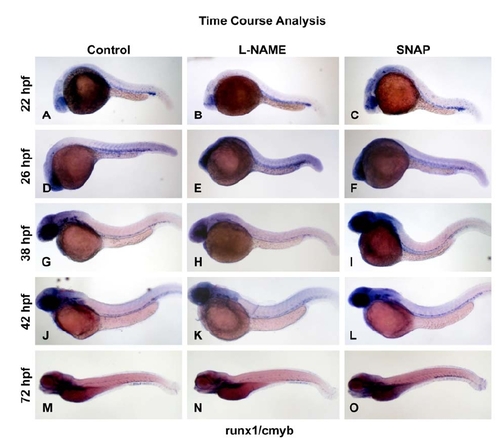
Time course analysis reveals time-specific effect of NO modulation on HSCs. |
|
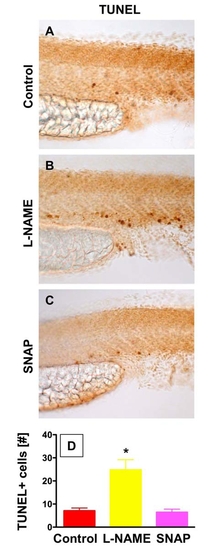
Nos inhibition increases apoptosis within the AGM |
|
NO signaling affects vascular and HSC development. |
|
The effect of nos1 MO inhibition is dose-dependent. |
|
Blastula transplant controls. |
|
NO modifies the effects of notch signaling on HSC formation. |
|
NICD-mediated elevation of ephB2 is blocked by nos inhibition. |
|
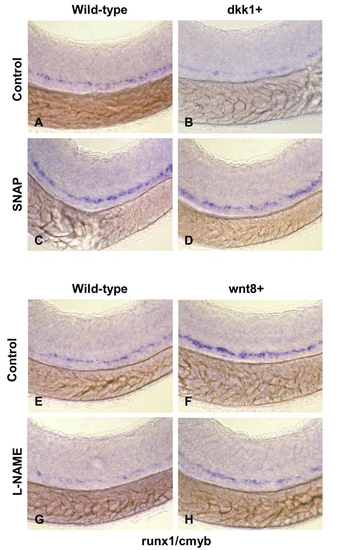
NO modifies the effects of wnt signaling on HSC formation. |
Reprinted from Cell, 137(4), North, T.E., Goessling, W., Peeters, M., Li, P., Ceol, C., Lord, A.M., Weber, G.J., Harris, J., Cutting, C.C., Huang, P., Dzierzak, E., and Zon, L.I., Hematopoietic stem cell development is dependent on blood flow, 736-748, Copyright (2009) with permission from Elsevier. Full text @ Cell