- Title
-
Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration
- Authors
- Goessling, W., North, T.E., Loewer, S., Lord, A.M., Lee, S., Stoick-Cooper, C.L., Weidinger, G., Puder, M., Daley, G.Q., Moon, R.T., and Zon, L.I.
- Source
- Full text @ Cell
|
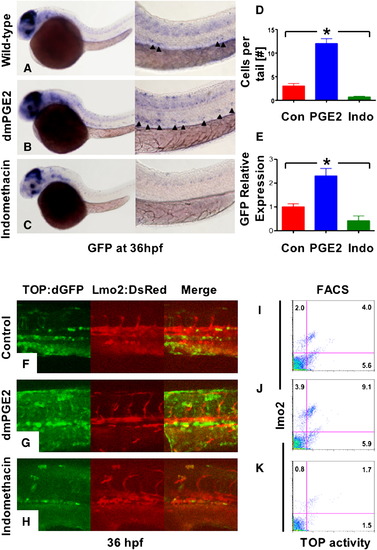
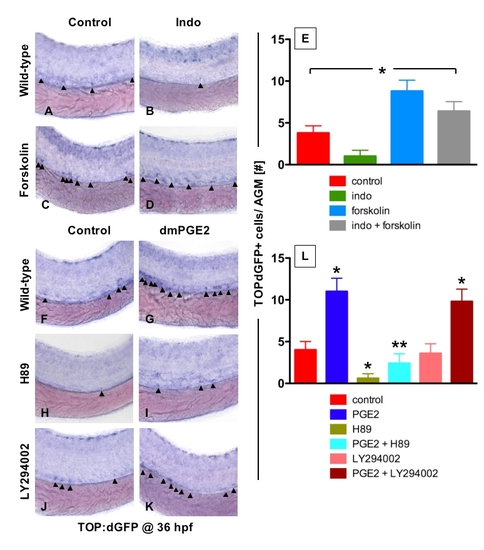
Prostaglandin Levels Directly Affect wnt Activity in Zebrafish Embryos (A–C) In situ hybridization for GFP in TOP:dGFP wnt reporter embryos at 36 hpf shows widespread wnt activity; inset, close-up of GFP+ (black arrowheads) cells in the AGM. 10 μM dmPGE2 enhanced GFP expression throughout the embryo, while 10 μM indo decreased global wnt activity, and in the AGM. (D) Quantification (mean ± SD) of total GFP+ cells in the major trunk vessels and (E) qPCR analysis for GFP in whole embryo lysates following exposure to dmPGE2 or indo versus vehicle con reveals significant alterations in wnt activity (*significant (sig) across treatment groups, ANOVA, p < 0.05, n = 10/treatment). (F–H) Representative confocal microscopy images of the AGM region of TOP:dGFP; lmo2:DsRed embryos following exposure to con, dmPGE2, or indo are shown; differences can be seen in the wnt-active (green, left column), HSC/endothelial (red, middle), and colocalized (merged, right) populations. (I–K) Representative FL1 (green)/ FL2 (red) FACS plots of individual TOP:dGFP; lmo2:DsRed embryos after exposure to con, dmPGE2 or indo confirm the confocal analyses (summarized in Figure S2). |
|
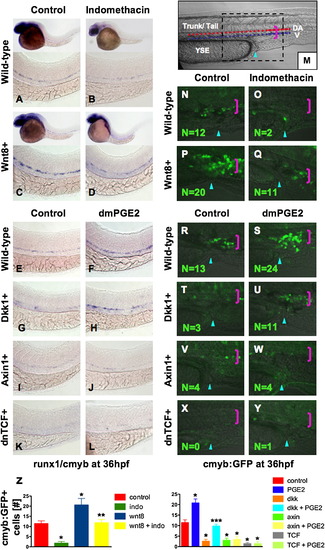
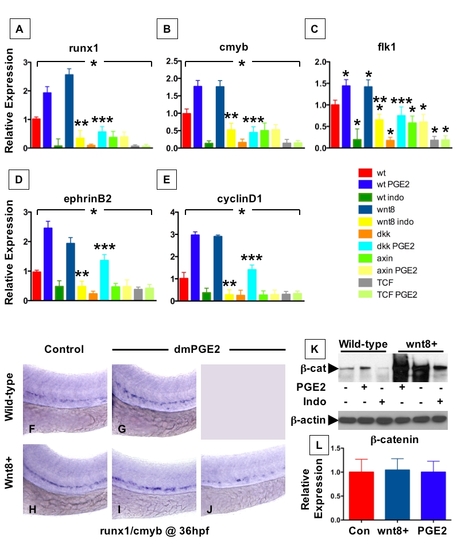
PGE2 Regulates Wnt-Mediated Effects on HSC Formation at the Level of β-catenin (A–D) wnt8 induction in hs:wnt8-GFP transgenic embryos increased runx1/cmyb+ HSCs compared to WT; indo decreased HSCs in WT and wnt8 embryos. (E–L) Induction of dkk diminished runx1/cmyb expression compared to WT. dmPGE2 enhanced HSCs in WT embryos, and rescued runx1/cmyb expression to approximately WT levels in dkk embryos. Axin (hs:axin-GFP) and dnTCF (hs:dnTCF-GFP) reduced runx1/cmyb expression, and dmPGE2 could not rescue those effects. (M) Schematic of confocal microscopy. Imaging was performed in the trunk/tail region of the embryo, centered around the tip of the yolk sac extension (YSE, blue arrowhead), encompassing the dorsal aorta (red dots) and vein (blue dots), as indicated by the pink bracket. (N-Y) In vivo confocal microscopy of wnt pathway inducible embryos crossed into the cmyb:GFP HSC reporter line confirmed the in situ hybridization analysis and demonstrated quantifiable effects on cell number. (Z) Cell counts (5 embryos/treatment, data represented as mean ± SD) were conducted in the major vessels (pink bracket) in a 40x field centered at the YSE; *sig versus con; **sig versus wnt8; ***sig versus dkk; ANOVA, p < 0.001. |
|
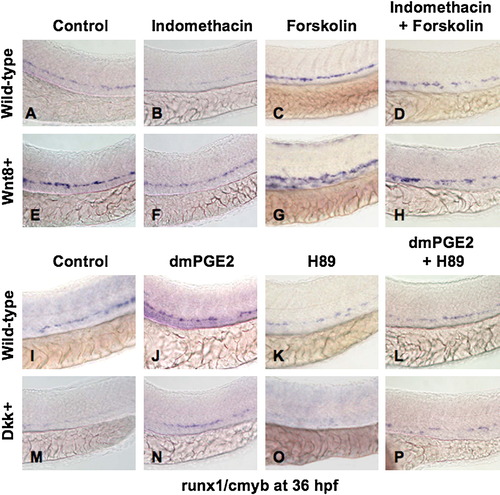
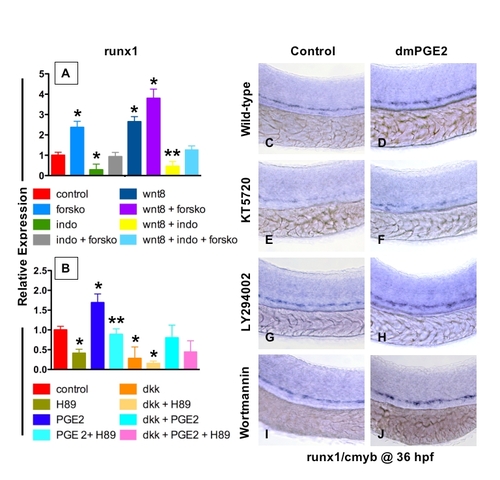
PGE2 Regulates the Effects of wnt Activity on HSCs via cAMP/PKA Signaling (A, B, E, F, I, J, M, and N) The /PGE2/wnt interaction affected runx1/cmyb+ HSC formation, as seen in Figure 2. (C and G) cAMP enhancement by forskolin increased runx1/cmyb expression in WT embryos and further expanded HSCs in wnt8 embryos. (D and H) Forskolin counteracted the inhibitory effect of indo on HSC formation in WT and wnt8 embryos. (K and O) PKA inhibition by H89 decreased runx1/cmyb expression in WT embryos and eradicated HSC formation in dkk embryos. (L and P) The enhancing effect of dmPGE2 on HSCs was reduced back to baseline levels by PKA inhibition; similarly, the dmPGE2-induced rescue of HSCs in dkk embryos was blocked by H89. |
|
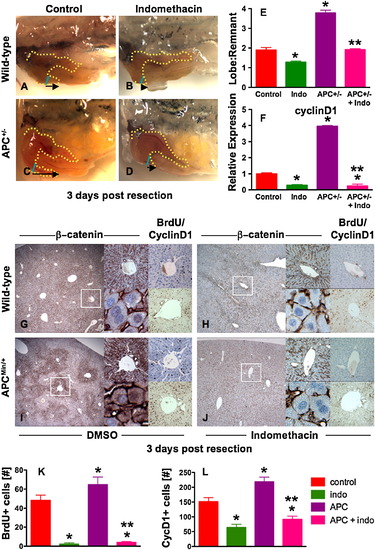
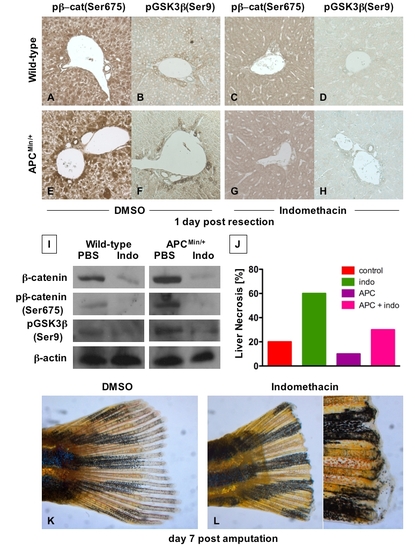
The PGE2/wnt Interaction Is a Master Regulator of Liver Regeneration (A–D) Representative photomicrographs of en bloc dissections following liver resections at day 3 are shown; the liver is highlighted by a yellow dotted line, the resection site by a blue line, and the black arrow indicates the amount of liver regrowth. Wnt activation in APC mutant zebrafish enhanced liver regeneration compared to WT. Indo stymied liver regeneration. (E) Quantification of zebrafish liver regeneration showed significant differences across treatment groups; *sig versus con, **sig versus APC+/-, ANOVA, p < 0.001, n ≥ 6. (F) qPCR for cyclin D1 gene expression; effects are coordinately regulated by the PGE2/wnt interaction; *sig versus con, **sig versus APC, ANOVA, p < 0.001, n = 7. (G–J) wnt and PGE2 modulation has significant effects on murine liver regeneration following 2/3 partial hepatectomy. APCMin mice exhibit enhanced β-catenin staining (left panel), particularly in the periportal areas (top middle panel), with noticeable nuclear staining (bottom middle panel). BrdU incorporation (top right panel) and cyclin D1 staining (bottom right panel) indicated enhanced regenerative activity. Indo diminished global β-catenin staining (left and top middle panels), excluded β-catenin from the nuclei (bottom middle panels), and resulted in a corresponding decrease of both BrdU incorporation and cyclin D1. (K and L) Quantification of BrdU incorporation and cyclinD1 staining in corresponding serial sections of regenerating livers; *sig versus con, **sig versus APC, ANOVA, p < 0.05, n = 10 sections/treatment. Data represented as mean ± SD. |
|
PGE2 affects the expression of HSC and vascular markers by increasing β-catenin levels |
|
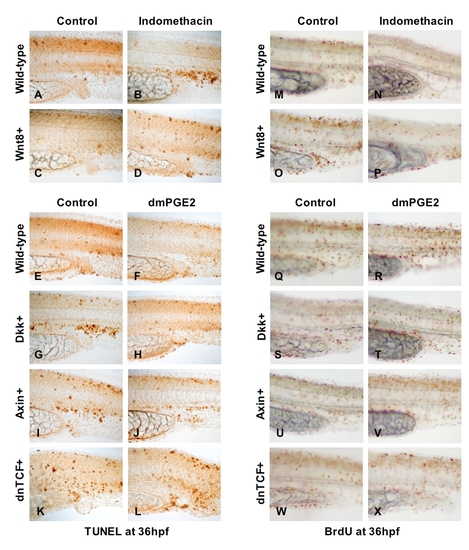
PGE2-mediated modulation of wnt signaling affects cell death and proliferation |
|
PGE2 enhances wnt activity in the HSC compartment via the PKA/cAMP pathway |
|
PGE2 enhances wnt activity in the HSC compartment via the PKA/cAMP pathway |
|
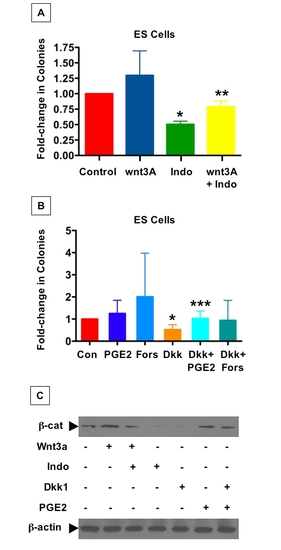
The PGE2/wnt interaction affects hematopoietic colony formation in murine ES cells |
|
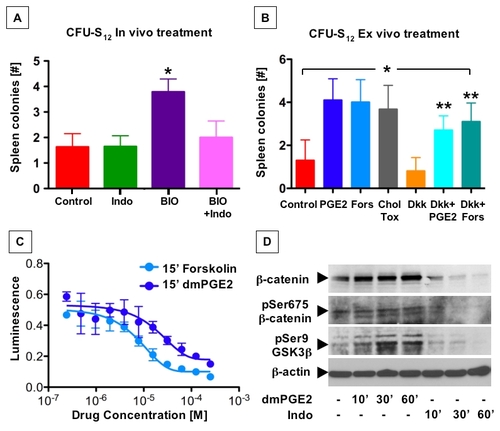
PGE2 affects hematopoietic progenitor function by enhanced cAMP production and subsequent phosphorylation events |
|
The interaction of PGE2 and wnt affects regeneration in several organs (A - H) Immunohistochemical analysis of liver samples 1 day post 2/3 partial hepatectomy in C57Bl/6 and APCMin mice. Photomicrographs were taken at 20x magnification. |
Reprinted from Cell, 136(6), Goessling, W., North, T.E., Loewer, S., Lord, A.M., Lee, S., Stoick-Cooper, C.L., Weidinger, G., Puder, M., Daley, G.Q., Moon, R.T., and Zon, L.I., Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration, 1136-1147, Copyright (2009) with permission from Elsevier. Full text @ Cell