- Title
-
Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration
- Authors
- Veldman, M.B., Bemben, M.A., Thompson, R.C., and Goldman, D.
- Source
- Full text @ Dev. Biol.
|
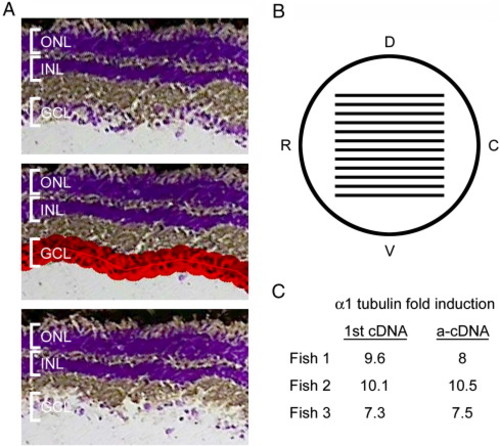
Laser capture microdissection (LCM) of RGCs and linear amplification of RNA isolated from these cells. (A) Images of a cresyl violet stained retinal section before RGC capture (top), with area to be captured highlighted in red (middle) and after RGC capture (bottom) LCM. (B) Schematic lateral view of the fish eye with lines representing the distribution of sections taken for RGC harvesting. Dorsal (D), ventral (V), rostral (R), and caudal (C). (C) Quantitative RT-PCR results from 1st strand cDNA and amplified cDNA (a-cDNA) showing that α1 tubulin was induced as expected in optic nerve-lesioned RGCs and that amplification was linear and accurately reflected α1 tubulin induction. These samples were used to prepare probes for the microarray hybridization. |
|
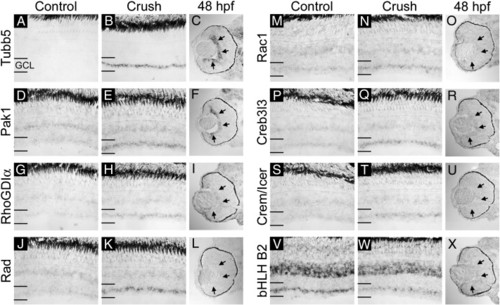
In situ hybridization verification of microarray results and comparison with expression in the developing retina (part I). mRNA for Tubb5 (A–C), Pak1 (D–F), RhoGDIα (G–I), Rad (J–L), Rac1 (M–O), Creb3l3 (P–R), Crem/Icer (S–U), and bHLHB2 (V–X) was detected by in situ hybridization in uninjured control, 3-day post-optic nerve crush and 48 hpf retinal sections. Note that mRNA levels are elevated in the ganglion cell layer (GCL) of Tubb5, Pak1, RhoGDIα, Rad, Rac1, Creb3l3, and Crem/Icer probed sections following nerve crush injury. bHLHB2 is decreased in the GCL following nerve crush injury. Developmentally, Tubb5, Pak1, RhoGDIα, and Creb3l3 are all expressed in the GCL at 48 hpf (arrows in panels C, F, I, and R). Rad and Crem/Icer are not expressed in the retina at 48 hpf (L and U). Rac1 and bHLHB2 are expressed weakly but uniformly in the retina at 48 hpf (O and X). Sense probes for all genes tested gave no specific signal (data not shown). |
|
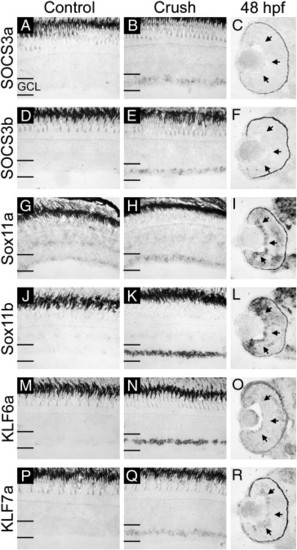
In situ hybridization verification of microarray results and comparison with expression in the developing retina (part II). mRNA for SOCS3a (A–C), SOCS3b (D–F), Sox11a (G–I), Sox11b (J–L), KLF6a (M–O), and KLF7a (P–R) was detected by in situ hybridization in uninjured control, 3-day post-optic nerve crush and 48 hpf retinal sections. Expression of all six genes is elevated in the ganglion cell layer (GCL) following optic nerve crush injury as compared to control. Developmentally, Sox11a, Sox11b, and KLF7a are expressed in the GCL at 48 hpf while SOCS3a, SOCS3b, and KLF6a are not (arrows in panels C, F, I, L, O, and R). Sense probes for all genes tested gave no specific signal (data not shown). |
|
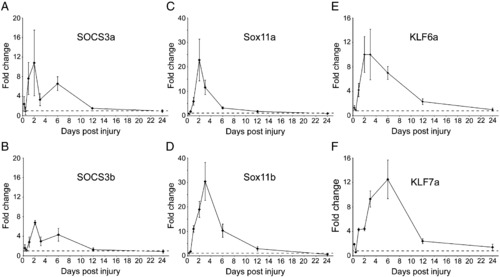
Quantitative RT-PCR time course of gene expression in the retina following optic nerve crush injury. Quantitative real-time RT-PCR was used to measure mRNA levels in the retina at various time points following optic nerve crush injury as compared to uninjured retina. Expression of GAPDH was used to normalize all samples. SOCS3a (A), SOCS3b (B), Sox11a (C), Sox11b (D), KLF6a (E), and KLF7a (F) are all induced by 1-day post-injury (dpi), peak around 2–6 dpi and return to basal levels by 12–24 dpi. The dotted line denotes basal uninjured levels in each graph. |
|
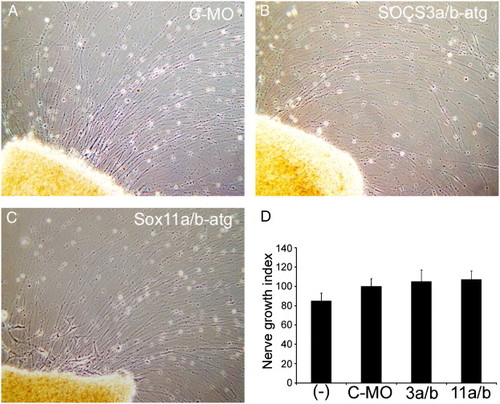
Knockdown of SOCS3a and SOCS3b or Sox11a and Sox11b has no effect on axon outgrowth in retinal explants. Morpholino-modified antisense oligonucleotides were delivered to RGCs in vivo via the sectioned optic nerve. Four days later, retinas were isolated, diced, and placed in explant culture for 4 days prior to analysis. Shown are representative images of axon outgrowth. Control morpholino-treated (A), SOCS3a and SOCS3b atg-targeting morpholino-treated (B), and Sox11a and Sox11b atg-targeting morpholino-treated explants (C) exhibit robust axon outgrowth after 4 days in culture. (D) Nerve Growth Index for explants from the indicated treatment groups: (-), no treatment (n = 23); C-MO, control morpholino (n = 34); 3a/b, SOCS3a/b-atg morpholino (n = 21); and 11a/b, Sox11a/b-atg morpholino (n = 68). Nerve Growth Index as determined by Landreth and Agranoff (1979) takes into account both axon length and density. Nerve Growth Index values were normalized to C-MO group that was defined as 100%. Error bars represent SEM. |
|
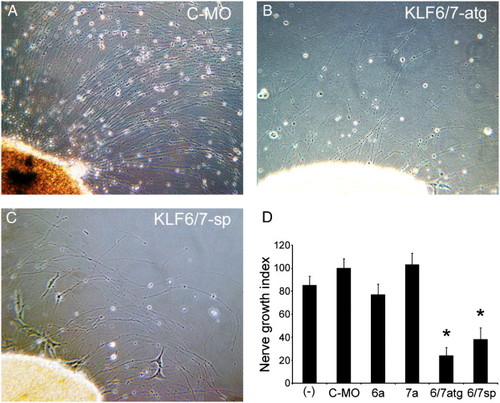
Knockdown of KLF6a and KLF7a together but not independently attenuates axon outgrowth from regenerating retinal explants. Morpholino-modified antisense oligonucleotides were delivered to RGCs in vivo via the sectioned optic nerve. Four days later, retinas were isolated, diced, and placed in explant culture for 4 days prior to analysis. Shown are representative images of axon outgrowth. Control morpholino-treated explants exhibit robust axon outgrowth (A) while explants treated with morpholinos targeting either the start codon of both KLF6a and KLF7a (B) or the splice junction of exon 5 of both KLF6a and KLF7a (C) exhibit shorter and fewer axons. (D) Nerve Growth Index for untreated explants (-), explants treated with individual morpholinos (C-MO, control morpholino; 6a, KLF6a-atg; or 7a, KLF7a-atg) or combined treatment to knockdown both KLF6a and KLF7a (6/7atg, KLF6a-atg + KLF7a-atg or 6/7sp, KLF6a-sp + KLF7a-sp). Combined knockdown of KLF6a and KLF7a significantly decreases axon outgrowth compared to control or single morpholino-treated explants. *p < 0.01, ANOVA with Bonferroni post-hoc test. Nerve Growth Index values were normalized to C-MO group that was defined as 100%. Error bars represent SEM. |
|
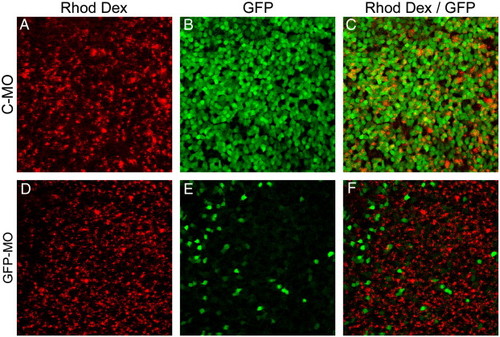
Morpholino-based gene knockdown is efficient in the adult retina. Gelfoam impregnated with morpholino and rhodamine dextran tracer were applied to the severed optic nerve of adult α1T-GFP transgenic fish. The fish were allowed to survive for 6 days at which point they were killed and the retinas isolated. Confocal images were then collected from flat mount retinas from these fish. Control morpholino (C-MO) (A, B, and C) had no effect on GFP expression from the α1T-GFP transgene following nerve injury. A morpholino designed to specifically target the transcription start site of the α1T-GFP transgene efficiently inhibits expression of GFP following nerve injury (D, E, and F). Note that the GFP-positive cells in panel E are rhodamine dextran negative (F), indicating they likely did not receive any morpholino. |
|
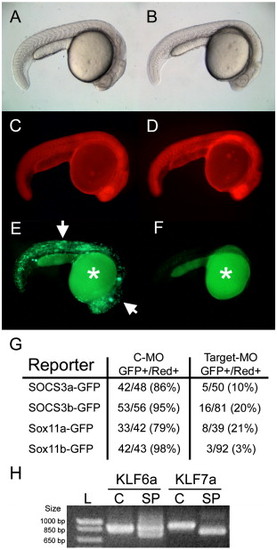
Morpholino specificity demonstrated by knockdown of GFP reporter expression or transcript mis-splicing in morpholino-treated embryos. Single cell zebrafish embryos were injected with morpholino, rhodamine dextran, and a GFP reporter plasmid and assayed for GFP expression 24 h later. (A and B) Bright-field images of 24 hpf embryos injected with control morpholino (C-MO) (A) or SOCS3a targeting morpholino (B) plus SOCS3a-GFP reporter plasmid. (C and D) Red fluorescence from rhodamine dextran demonstrating efficient injection in both embryos. (E and F) GFP expression is present in many cells of the C-MO-injected embryo (arrows in E) while GFP is absent in the target morpholino injected embryo (F). Auto-fluorescence present in the yolk is denoted by an asterisk. (G) Tabular results of embryo counts for each reporter and morpholino used in this study. In all cases, the C-MO had little effect on reporter expression while each targeting morpholino significantly decreased the number of GFP-positive embryos. (H) Single cell embryos were injected with control (C) or KLF splice-site targeted (SP) morpholino and RNA isolated 24 h later for analysis of mis-splicing using RT-PCR. Note that the splice site targeted morpholinos resulted in mis-splicing of each gene with mis-splicing of KLF7a being complete and mis-splicing of KLF6a being partial. |
Reprinted from Developmental Biology, 312(2), Veldman, M.B., Bemben, M.A., Thompson, R.C., and Goldman, D., Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration, 596-612, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.