PUBLICATION
Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion
- Authors
- Gamble, J.T., Reed-Harris, Y., Barton, C.L., La Du, J., Tanguay, R., Greenwood, J.A.
- ID
- ZDB-PUB-181106-2
- Date
- 2018
- Source
- Biochemical and Biophysical Research Communications 506(4): 833-839 (Journal)
- Registered Authors
- Barton, Carrie, La Du, Jane K., Tanguay, Robyn L.
- Keywords
- Glioblastoma, ImageJ, Invasion, Lama5, Laminin alpha 5, Zebrafish
- MeSH Terms
-
- Neoplasm Invasiveness
- Glioblastoma/metabolism*
- Glioblastoma/pathology*
- Morpholinos/pharmacology
- Cell Line, Tumor
- PubMed
- 30389143 Full text @ Biochem. Biophys. Res. Commun.
Abstract
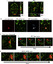
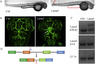
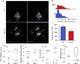
Glioblastoma (GBM) is a deadly disease due to its ability to quickly invade and destroy brain tissue. Slowing or stopping GBM cell progression is crucial to help those inflicted with the disease. Our lab created an embryo-larval zebrafish xenograft model as a tool to study human GBM progression in an observable brain environment. The zebrafish brain is a dynamic and complex environment providing an optimal setting for studying GBM cell progression. Here we demonstrate the ability of our model to quantitate GBM proliferation, dispersal, blood vessel association, microtumor formation, and individual cell invasion by evaluating the importance of an extracellular matrix protein, laminin alpha 5 (lama5), on U251MG cell progression. Lama5 has been implicated in cancer cell survival, proliferation and invasion and is a known adhesion site for GBM cells. While lama5 is highly expressed in endothelial cells in the brain, it is unknown how lama5 affects GBM behavior. Using a lama5 morpholino, we discovered that lama5 decreased U251MG dispersal by 23% and doubles the formation of blood vessel dependent microtumors. Despite lama5 being a known attachment site for GBM, lama5 expression had no effect on U251MG association with blood vessels. Analysis of individual U251MG cells revealed lama5 significantly lowered invasion as mobile U251MG cells traveled 32.5 μm less, invaded 5.0 μm/hr slower and initiated invasion 60% few times per cell.
Genes / Markers
Expression
Phenotype
Mutations / Transgenics
Human Disease / Model
Sequence Targeting Reagents
Fish
Orthology
Engineered Foreign Genes
Mapping