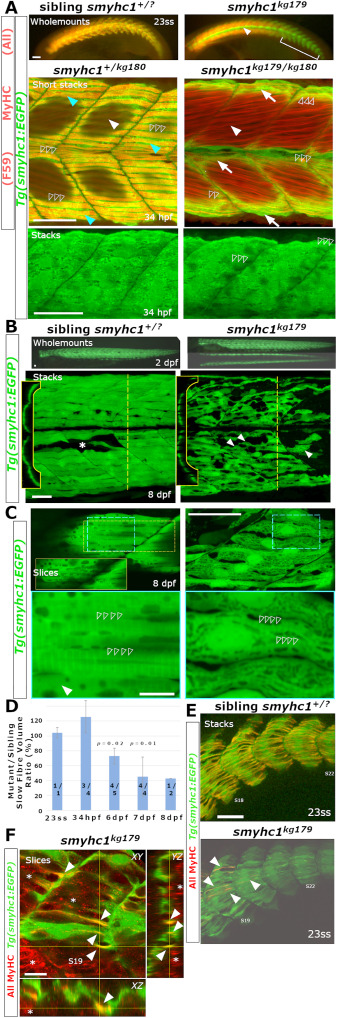
Fig. 5 Survival of SSFs without Smyhc1. Sibling zebrafish from smyhc1kg179/+;Tg(smyhc1:EGFP)i104 male crossed to a female smyhc1kg180/kg180 or smyhc1kg179/+;Tg(smyhc1:EGFP)i104 mounted dorsal up, anterior to left, shown in live wholemount or confocal maximal intensity projection stacks or slices. A) Upper panels: 23ss embryos stained for sarcomeric MyHC revealing absence of signal in slow fibres marked by GFP in mutant. Note the absence of MyHC in muscle pioneers (arrowhead) and slow fibres in tail regions lacking fast MyHC (bracket). Middle panels: Short stacks of somite 19/20 stained for MyHC with F59 showing the presence of a layer of SSFs marked by EGFP (arrows) in both mutant and sibling at 34 hpf. Note the coincidence of strong slow MyHC and EGFP in SSFs (cyan arrowheads) only in the sibling. Dark areas reflect regions of the stack containing underlying fast fibres. The short stack in the mutant was selected to show that MyHC is also weakly detected by F59 in obliquely-orientated fast fibres (white arrowheads). EGFP signal in SSFs of mutant reveals a striated sarcomere-like pattern despite the absence of MyHC (open arrowheads). Lower panels: Magnified full stacks of the same embryos show EGFP in dorsal myotomes of somites 18–20 revealing normal SSF orientation, nuclear positioning and striation (open arrowheads) in mutant. B) Live smyhc1kg179;Tg(smyhc1:EGFP) mutant and sibling embryos/larvae. Upper panels: Lateral views, with dorsal view below, showing that all SSFs have migrated to the lateral myotome in a 2 dpf mutant. Lower panels: Live 8 dpf larval somites 17–19 showing migrated mutant SSFs detached from the vertical myosepta (arrowheads), compared with a rare fibre defect in the SSF layer in sibling (asterisk). Insets show reduced volume of mutant SSFs in transverse optical sections at the dashed lines. C) Single confocal slices of EGFP in SSF layers with blue boxes magnified below. Whereas the sibling had cytoplasm largely filled with myofibrils containing a regular 1.96 μm sarcomeric array (open arrowheads), the mutant showed prominent nuclei, a thin disorganized cytoplasm filled with vacuoles between which ran immature and poorly-aligned material with closer striations (open arrowheads). Some fibre regions in sibling were devoid of myofibrils (arrowhead) and contained prominent and extensive vacuoles (Inset yellow box is a slice 5 μm more superficial to the dashed yellow box region displaying the abundant vacuolar structures at the myotome surface that are less apparent deeper within the SSF layer. D) Quantification of gradually reduced EGFP cell volume of SSFs in smyhc1kg179;Tg(smyhc1:EGFP) mutants compared to their siblings at the same age. Mean (± SEM when N allowed, calculated by to include propagation of error in both sibling and mutant measures) of the proportion for scanned segment of at least three somites centred on somite 18. On each column, fraction is number of mutant/sibling fish analysed with identical scan and post-processing parameters. Thus, at 1–2 dpf four embryos had SSFs of similar volume to those in five of their non-mutant siblings whereas, at 6–8 dpf, nine mutant larvae had SSFs about half the size of those in 11 non-mutant siblings. P-values above columns represent t-test on measured volumes of mutants versus siblings at each age. E,F). Confocal stacks (E) and slices (F) of 2 h-old somites at 23ss showing the emergence of small MyHC-containing myofibrils in mutant SSFs (arrowheads). Crosshairs in F indicate slice planes. Asterisks mark the initiation of deep fast MyHC accumulation in more anterior somites. Bars 50 μm (A,B,C upper, E) and 10 μm (C lower, F).
Reprinted from Developmental Biology, 499, Hau, H.A., Kelu, J.J., Ochala, J., Hughes, S.M., Slow myosin heavy chain 1 is required for slow myofibril and muscle fibre growth but not for myofibril initiation, 47-58, Copyright (2023) with permission from Elsevier. Full text @ Dev. Biol.