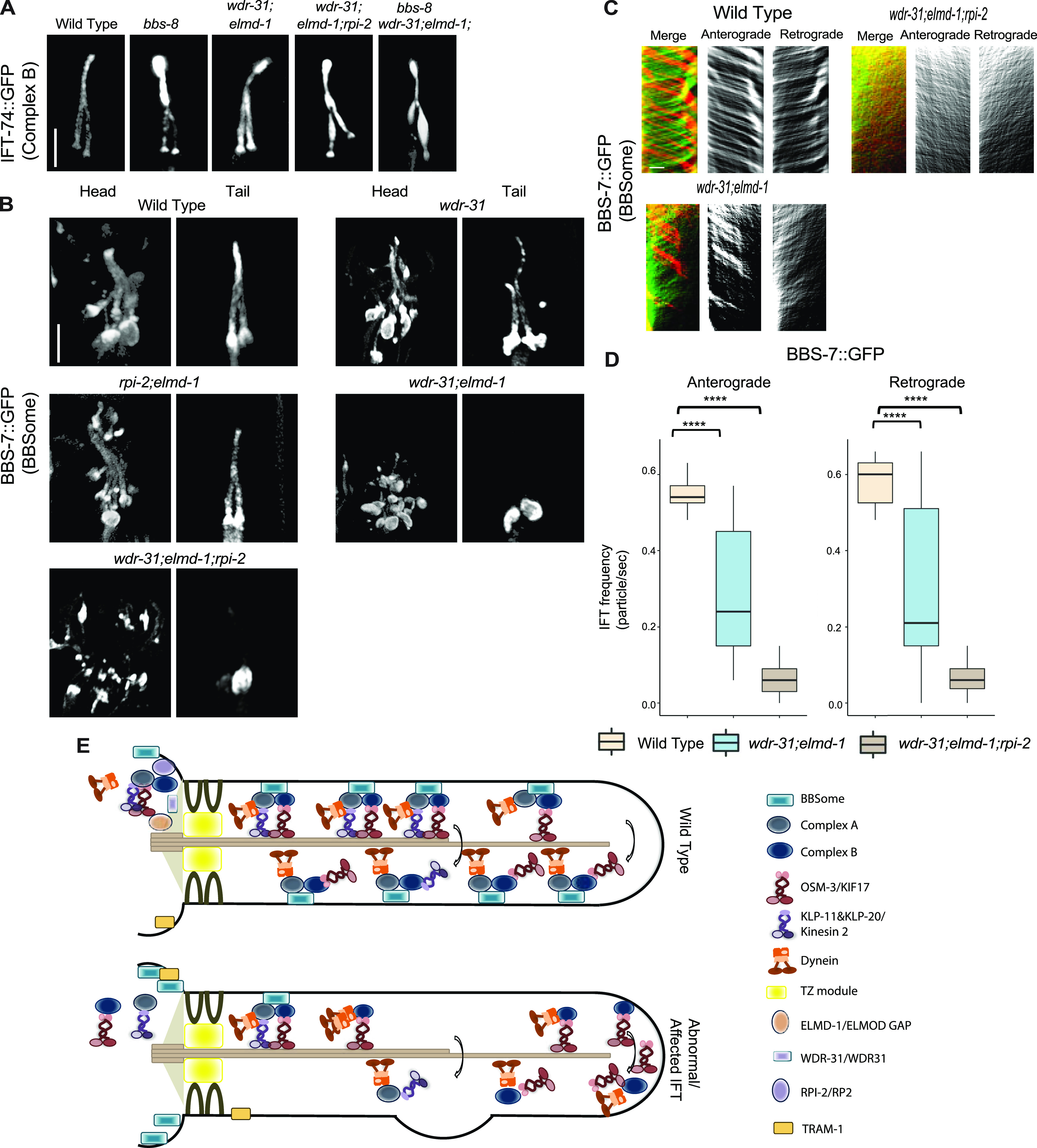
Figure 9.
(A) Shown are fluorescence images from the transgenic strain carrying IFT-74::GFP, an IFT-B component, in WT, wdr-31;elmd-1 double mutants, wdr-31;elmd-1;rpi-2 and wdr-31;elmd-1;bbs-8 triple mutants, and bbs-8(nx77). The IFT-B subunit IFT-74:GFP accumulates at the ciliary tips and cilia in the tails of all three mutants. (B) Confocal fluorescence images showing the localization of BBS-7:GFP, a BBSome subunit, in the heads and tails of WT and wdr-31(tm10423); wdr-31;elmd-1; rpi-2;elmd-1 double and wdr-31;elmd-1;rpi-2 triple mutants. Fluorescence images showed absent or weak cilia staining of BBS-7::GFP in both the heads and tails of wdr-31;elmd-1 and wdr-31;elmd-1;rpi-2 triple mutants. (C) Kymographs were created from time-lapse BBS-7::GFP movies (PHA/PHB cilia) using KymographClear integrated into ImageJ. Shown are representative kymographs for BBS-7::GFP translocating in WT and indicated mutants. Each trajectory in kymographs was counted. (D) Travel time and distance are included on kymograph (D) The graph depicts the average number of BBS-7::GFP particles traveling around the cilia in both directions for WT and indicated mutants. (E) The Mann–Whitney U test revealed statistical significance between the compared strains and that the P-value was less than 0.0001 shown by the four asterisks (****) at the top of the brackets (E) In WT, the assembly of the Kinesin–IFT-–BBSome complex (Kinesin-II and OSM-3, IFT-B, IFT-A, and BBSome) happens at the base of the cilia. In the middle segment of amphid and phasmid cilia in C. elegans, both heterotrimeric Kinesin II and homodimeric OSM-3 transport the IFT-A–IFT-B–BBSome complex in an anterograde direction. Heterotrimeric Kinesin II returns to the ciliary base when it reaches the tip of the middle segment of amphid and phasmid cilia, whereas homodimeric OSM-3 is responsible for the anterograde translocation of the IFT–BBSome complex in the distal segment of amphid and phasmid cilia. When the OSM-3–IFT–BBSome complex reaches the ciliary tip, cytoplasmic dynein transports them back to the ciliary base. In wdr-31;elmd-1 double wdr-31;elmd-1;rpi-2 triple mutants, the BBSome failed to enter into the cilia, thus leading to accumulations of OSM-3 and IFT-B components in the ciliary tips.