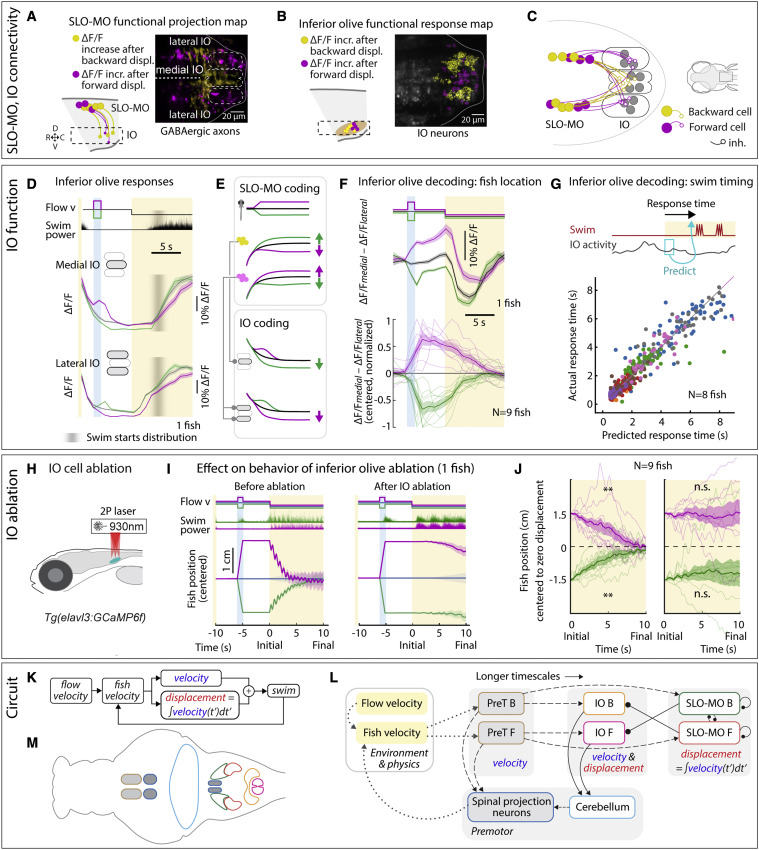
Fig. 6 IO is functionally downstream of SLO-MO and necessary for positional homeostasis
(A) Imaging projections from SLO-MO in IO area in Tg(gad1b:Gal4; UAS:GCaMP6f; elavl3:H2B-jRGECO1b). The gad1b channel shows anatomical segregation of projections of SLO-MO neurons that encode forward pre-displacements (projections to medial IO) versus backward pre-displacements (projections to lateral IO). Magenta (yellow) stands for forward-pre-displacement-positive (backward-pre-displacement-positive) SLO-MO cells.
(B) Imaging IO neurons (same fish as in A; pan-neuronal channel shown) showing segregation of neurons responding positively to forward pre-displacements (medial IO) versus backward pre-displacements (lateral IO)—the reciprocal of (A), consistent with inhibition of IO by SLO-MO axons. Magenta (yellow) stands for forward displacements (backward displacements) of fish in VR.
(C) Hypothesized connectivity between SLO-MO and IO based on (A) and (B).
(D) Time courses of IO responses to location changes. Medial IO is persistently suppressed relative to no pre-displacement following backward pre-displacements. Lateral IO is persistently suppressed relative to no pre-displacement following forward pre-displacements. The initial high ΔF/F levels are carried over from the end of the previous swim period (example fish, population data shown in Figures S7N and S7O).
(E) Schematic of location coding in IO versus SLO-MO and connectivity.
(F) Fish location can be decoded from instantaneous IO activity by taking the difference between medial and lateral IO signals.
(G) Predicting time of first swim bout during the swim period from IO activity at onset of the swim period (before first bout) across 8 fish. Swim time can be predicted, consistent with premotor role of IO.
(H) Schematic of two-photon IO cell ablation.
(I) Example fish positional homeostasis before IO ablation showing intact location memory, and same fish after IO ablation showing a complete loss of location memory.
(J) Population data before and after IO ablation, showing consistent loss of ability to correct for positional shifts after ablation. Animals still swim in response to instantaneous flow, but memory expression is lost. Thus, IO is necessary for self-location memory and accurate positional homeostasis. (9 fish, Wilcoxon sign-rank test, ∗∗p < 0.01, p = 7.6e−3.)
(K) Diagram of simplified control system for positional homeostasis, also used to simulate Figure 1D, bottom.
(L) Diagram of hypothesized brain-wide functional circuit. Dotted gray lines: interactions between the brain and environment. Solid black lines: connectivity within the discovered multiregional hindbrain circuit that mediates location memory. Dashed black lines: direct or indirect connections between this circuit and candidate visual and premotor regions. Round head arrows, inhibitory connections; PT, pretectum; IO, inferior olive; SLO-MO, spatial location encoding medulla oblongata neurons. Colors correspond to the annotations in (M).
(M) Approximate anatomical locations of circuit elements diagrammed in (L).
Reprinted from Cell, 185, Yang, E., Zwart, M.F., James, B., Rubinov, M., Wei, Z., Narayan, S., Vladimirov, N., Mensh, B.D., Fitzgerald, J.E., Ahrens, M.B., A brainstem integrator for self-location memory and positional homeostasis in zebrafish, 50115027.e205011-5027.e20, Copyright (2022) with permission from Elsevier. Full text @ Cell