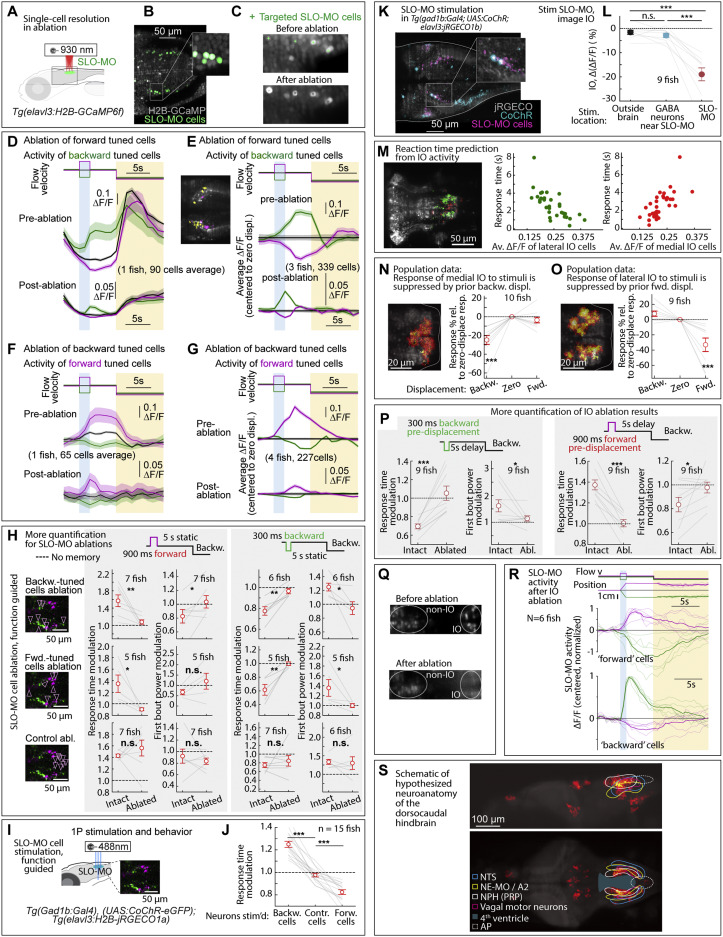
Fig. S7 Additional ablation and stimulation results, and dorsocaudal hindbrain anatomy, related to Figures 2, 5, and 6
(A) Ablation setup for ablating functionally identified SLO-MO populations using a two-photon laser and Tg(elavl3:GCaMP6f) fish.
(B and C) Calcium imaging of functionally identified and targeted SLO-MO cells before and after two-photon ablation showing single-cell precision.
(D) SLO-MO cells (of one example fish) whose activity increases after backward pre-displacement, before and after ablation of SLO-MO cells whose activity increases after forward pre-displacement. A sensory response remains, but persistence is abolished.
(E) Population data, similar to (D), showing the loss of persistent activity but preservation of sensory responses across 3 fish. Inset: example single imaging plane showing some spatial intermingling of forward-tuned SLO-MO cells (magenta) and backward-tuned SLO-MO cells (yellow).
(F and G) Similar to (D) and (E), but showing activity of forward-tuned cells before and after ablation of backward-tuned cells, again showing the loss of persistence but preservation of sensory responses.
(H) Additional quantification of the effects of SLO-MO ablation. Ablation of backward-tuned cells, ablation of forward-tuned cells, and control ablations are shown. (Two-tailed paired t test, ∗∗p < 0.01, ∗p < 0.05, n.s. p > 0.05. Error bars: SEM in all panels.)
(I) Diagram of optogenetic stimulation and functional imaging setup.
(J) Optogenetic excitation of functionally identified SLO-MO neurons causes changes in the time to first swim bout in the swim period, consistent with changes in distance swum shown in Figures 5G and 5H. (Two-tailed paired t test, ∗∗∗p < 0.001.)
(K) Functionally identified SLO-MO neurons in Tg(gad1b:Gal4; UAS:CoChR; elavl3:jRGECO1b) fish from the elavl3:jRGECO1b red channel overlaid with the gad1b:Gal4; UAS:CoChR green channel. Single-cell specificity is obtained by sparse expression of CoChR (using the Gal4-UAS system) and stimulation with a high-resolution DMD setup.123
(L) Optogenetic stimulation of SLO-MO neurons (one neuron at a time of any functional type; hence no significant integration expected) causes inhibition of specific IO neurons (population data). Stimulation of CoChR-positive non-SLO-MO neurons causes no significant IO response. (p = 1.7e−7 by one-way ANOVA, 9 fish. ∗∗∗p < 0.001, by Tukey’s post hoc test. Same data as Figure 2K. Shaded regions and error bars: SEM in all panels.)
(M) Correlation between lateral and medial IO activity and reaction time, complementing the joint decoder of Figure 6G.
(N and O) Backward displacements suppress medial IO activity; forward displacements suppress lateral IO activity. Population data to complement Figures 6A–6D. (One-sample t test, ∗∗∗p < 0.001.)
(P) Additional quantification of effects of IO lesions on response time and swim power. (9 fish, two-tailed paired t test, ∗∗∗p < 0.001, ∗p < 0.05. Error bars: SEM in all panels.)
(Q) Calcium imaging of IO neurons using Tg(elavl3:H2B-GCaMP6f) fish before and after IO ablation.
(R) Persistent SLO-MO activity encoding self-location remains intact after ablation of IO cells.
(S) Known and hypothesized neuroanatomy of the dorsocaudal larval zebrafish hindbrain. This schematic, based on multiple zebrafish and mouse publications and atlases, accompanies the hypothesis that SLO-MO overlaps with the homolog of the nucleus prepositus hypoglossi (NPH), a major GABAergic input to the IO.94,95 SLO-MO neurons are also GABAergic and project to and inhibit the IO. Our results are consistent with a monosynaptic connection between SLO-MO and IO, and we showed that activating SLO-MO functionally inhibits IO. SLO-MO neurons are spatially arranged relative to the nucleus of the solitary tract (NTS), noradrenergic area A296 (called NE-MO in Mu et al.52), and are close to the fourth ventricle 97; the NPH also has these properties (Allen Mouse Brain Atlas, 93). Historically, the NPH has been studied in the context of eye movement control, but (parts of) the NPH may be multifunctional. Circuits for positional homeostasis and for oculomotor control have in common that they must integrate visual slip into a persistent signal,124, 125, 126,so the possibility exists that these circuits showed some overlap, possibly with similar mechanisms for persistent activity.
Reprinted from Cell, 185, Yang, E., Zwart, M.F., James, B., Rubinov, M., Wei, Z., Narayan, S., Vladimirov, N., Mensh, B.D., Fitzgerald, J.E., Ahrens, M.B., A brainstem integrator for self-location memory and positional homeostasis in zebrafish, 50115027.e205011-5027.e20, Copyright (2022) with permission from Elsevier. Full text @ Cell