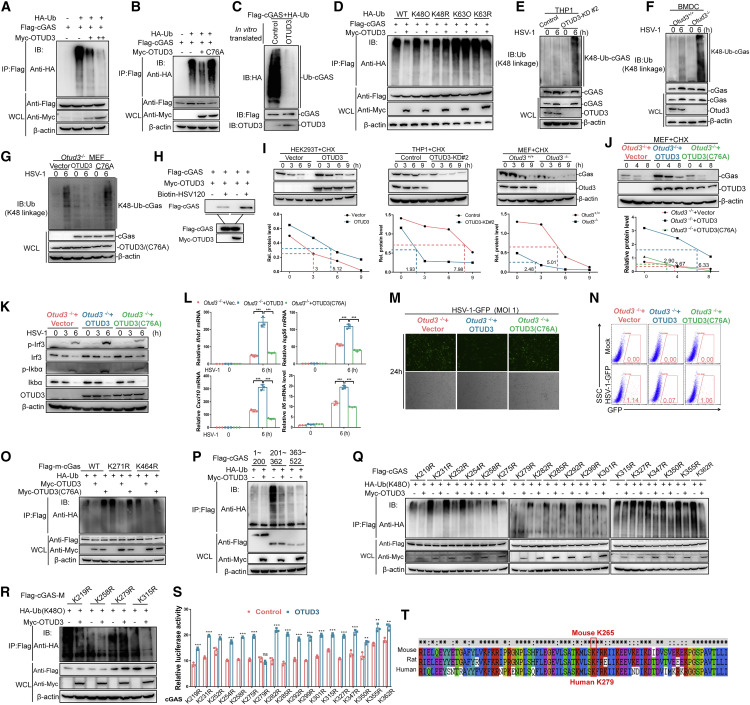
Fig. 7 Figure 7. OTUD3 deubiquitinates cGAS at Lys279 by removing K48-linked ubiquitin chain (A) IB for polyubiquitination of cGAS in HEK293T cells transfected with the indicated expression plasmids, FLAG-cGAS, HA-Ub, and increasing amount of Myc-OTUD3, and followed by immunoprecipitating with anti-FLAG beads. (B) IB for polyubiquitination of cGAS in HEK293T cells transfected the indicated expression plasmids, FLAG-cGAS, HA-Ub, and Myc-OTUD3 or Myc-OTUD3 (C76A) (an enzymatic inactive form of OTUD3) for 24 h, followed by pull-down assays with anti-FLAG antibody-conjugated agarose beads. (C) IB for polyubiquitination of cGAS in vitro. Ubiquitinated cGAS was eluted from anti-FLAG precipitated-HEK293T cell lysates transfected with FLAG-cGAS (5 μg) and HA-ubiquitin (5 μg), which were incubated with in vitro-translated OTUD3 or empty control. (D) IB for polyubiquitination of cGAS in HEK293T cells transfected with the indicated expression plasmids, FLAG-cGAS, HA-tagged ubiquitin (WT-Ub), or HA-tagged K48-only ubiquitin (K48O), or HA-tagged K63-only ubiquitin (K63O), or HA-tagged K48R ubiquitin (K48R), or HA-tagged K63R ubiquitin (K63R), together with the empty vector or Myc-OTUD3, followed by immunoprecipitating with anti-FLAG antibody-conjugated agarose beads. (E) IB for endogenous polyubiquitination of cGAS in the control and OTUD3-knockdown THP-1 cells infected with HSV-1 for 6 h. (F) IB for endogenous polyubiquitination of cGAS in Otud3+/+ and Otud3−/− BMDCs (1 × 108) infected with HSV-1 for 6 h. (G) IB for endogenous K48-linked polyubiquitination of cGAS in Otud3−/− MEFs reconstituted with the empty vector, OTUD3, or OTUD3 (C76A) followed by the un-infection or infection of HSV-1 for 6 h. (H) The effect of OTUD3 on the binding ability of cGAS to dsDNA. HEK293T cells were transfected with the indicated plasmids for 20 h. The cell lysates were then incubated with biotinylated HSV120 and streptavidin-Sepharose beads. The bead-bound proteins were analyzed by immunoblotting with the indicated antibodies. (I) IB of cGAS, OTUD3, and β-actin (upper blots) and quantitative analysis of the intensity of cGAS (relative to β-actin) (lower graphs) in HEK293T cells, THP-1, and MEFs in the presence or absence of CHX for 0–9 h. (J) IB of cGAS, OTUD3, and β-actin (left blots) and quantitative analysis of the intensity of cGAS (relative to β-actin) (right graphs) in Otud3−/− MEFs reconstituted with the empty vector, OTUD3, or OTUD3 (C76A) in the presence or absence of CHX for 0–8 h. (K) IB of proteins in Otud3−/− MEFs reconstituted with the empty vector, OTUD3, or OTUD3 (C76A) followed by the un-infection or infection of HSV-1 for the indicated times. (L) qRT-PCR analysis of Ifnb1, Isg56, Cxcl10, and Il6, in Otud3−/− MEFs reconstituted with the empty vector, OTUD3, or OTUD3 (C76A), followed by the infection of HSV-1 for 0–6 h. (M and N) Fluorescent microscopy imaging and flow cytometry analysis of the replication of HSV-GFP in Otud3−/− MEFs reconstituted with the empty vector, OTUD3, or OTUD3 (C76A) followed by HSV-GFP challenge (1 MOI) for 24 h. (O) IB for polyubiquitination of mouse cGAS (m-GAS) or mouse cGAS mutants (K271R and K464R) in HEK293T cells transfected with the indicated expression plasmids, HA-Ub, FLAG-mouse-cGAS (m-cGAS), or FLAG-mouse-cGAS mutants (K271R and K464R) together with empty vector, Myc-OTUD3, or Myc-OTUD3 (C76A) for 24 h followed by immunoprecipitating with anti-FLAG antibody-conjugated agarose beads. (P) IB for polyubiquitination of cGAS domains in HEK293T cells transfected with the indicated expression plasmids, FLAG-tagged truncated cGAS mutants (1–200 aa, 201–362 aa (M) and 363–522 aa), together with FLAG-MDA5, Myc-OTUD3, and HA-ubiquitin for 24 h followed by immunoprecipitating with anti-FLAG antibody-conjugated agarose beads. (Q) IB for K48-linked polyubiquitination of various mutants of cGAS in HEK293T cells transfected with the indicated expression plasmids, Myc- OTUD3, HA-Ub (K48O), or FLAG-tagged cGAS mutants (in which lysine was substituted with arginine individually) for 24 h followed by immunoprecipitating with anti-FLAG antibody-conjugated agarose beads. (R) IB for K48-linked polyubiquitination of four mutants of cGAS in HEK293T cells transfected with the indicated expression plasmids, Myc-OTUD3, HA-Ub (K48O), or FLAG-tagged cGAS domain (FLAG-cGAS-M) mutants (K219R, K258R, K279R, or K315R) for 24 h followed by immunoprecipitating with anti-FLAG antibody-conjugated agarose beads. (S) Luciferase activity of IFN-β promoter luciferase reporter in HEK293T cells transfected FLAG-cGAS mutants together with Myc-OTUD3. (T) Alignment of amino acid sequences around human Lys279 from mouse, rat, and human. The conserved lysine residue is circled by a red rectangle. ns, no significance, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, using unpaired Student’s t test (G) or two-way ANOVA with Holm-Sidak’s multiple comparisons test (I, M, and T). Data based on one representative experiment performed in three biological replicates from at least three independent experiments (mean ± SD) or representative data (A–F, H, J–L, and N–S).
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Cell Rep.