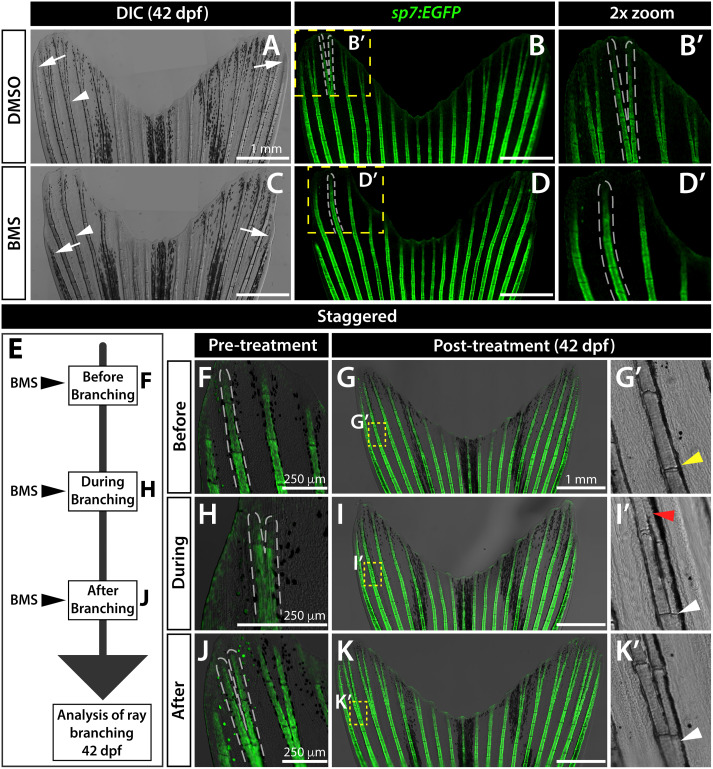
Fig. 3 Sustained Shh/Smo signaling is required for ray branching in developing caudal fins. (A–D) Differential interference contrast (DIC) and fluorescence images of caudal fins from DMSO (A, B) and BMS-833923 (BMS)-treated (C, D) 42 dp f sp7:EGFP osteoblast reporter fish. Dashed white lines outline dorsal ray 3 and daughter rays, when present. White arrowheads designate a present (A) or absent (B) branch point. White arrows mark ends of the principal peripheral rays. (E–K) Experimental schematic of (E) and resulting caudal fin images of 42 dpf sp7:EGFP fish from (F–K’) staggering the start of BMS treatment to before (F-G’), during (H–I’), and after branch initiation (J-K’). Yellow dashed boxes outline dorsal ray 2 regions shown in (G’, I’ and K’). The yellow arrowhead in (G’) designates where branching would have occurred without Shh/Smo-inhibition. The red arrowhead in (I’) marks where a ray re-fused when BMS was added after branching had initiated. White arrowheads in (I’ and K’) indicate ray branch points. Images represent treatment groups (before, during, and after) of n=4 or 5 BMS-treated and n = 2 or 3 DMSO controls. Ray re-fusions occur in 4 of 5 fish treated with BMS “during” ray branching in rays 2, 3, and 16. Scale bars are 250 μm or 1 mm, as indicated.
Reprinted from Developmental Biology, 477, Braunstein, J.A., Robbins, A.E., Stewart, S., Stankunas, K., Basal epidermis collective migration and local sonic hedgehog signaling promote skeletal branching morphogenesis in zebrafish fins, 177-190, Copyright (2021) with permission from Elsevier. Full text @ Dev. Biol.