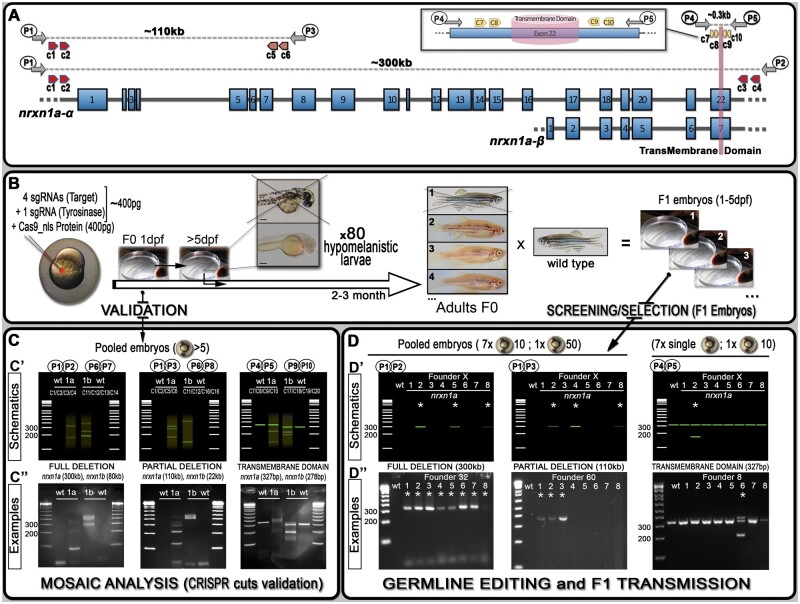
Figure 1
Schematics of neurexin organization and pipeline for generating mutants with large deletions. (A) Representation of neurexin1a (nrxn1a) genomic region together with sgRNA (CRISPR) target sites (c1–10) as well as primers used for validation and screening. The size of exons, intron gaps, and protein domains (and compared with one another) are not drawn to scale. (B) Pipeline designed to generate large deletions in zebrafish; 24 h post-injection, pools of five embryos are collected to evaluate the efficiency of the Cas9/sgRNAs mixes (see (C) mosaic analysis). Following validation, 80× 5dpf-larvae are selected based on their pigmentation phenotype. Once adult, F0 animals are crossed against wild-type to identify carriers/founders able to transmit the designed deletion to F1 mutants (see (D) stable validation). (C) To analyze/validate the efficiency of our sgRNAs-mixes, we proceeded with a basic PCR approach. Depending on the size of the target, a wild-type amplicon may be amplified; with the presence of a wild-type product hampering the amplification/detection of DNA remodeling (especially if rare). If large DNA deletion(s) happens, one should observe smearing with the presence of clear amplicon(s), as presented in the C’ schematics. The lower panel (C”) present the results we obtained for nrxn1a and nrxn1b transient validations. These transient validations can also be performed on individual embryos. (D) The same PCR approach is conducted to identify F0 adult founders. This time, eight batches are collected as schematically described. As opposed to the mosaic analysis (transient approach), a sharp band(s) should be observed, evidencing the presence of a large deletion. Upper panels (D’) represent theoretical results for one specific founder, while lower panels (D”) present PCR profiles obtained during our screen against the different nrxn1a deletion design. Founder frequency was evaluated at approximately 1/10. Asterisks* highlight positive founders carrying the desired mutation. All sequencing data as well as alignments and annotated gene files are available in the Supplementary zip file S04 with mutations summarized in Supplementary Table S04. A step-by-step protocol is also available in Supplementary file S05.