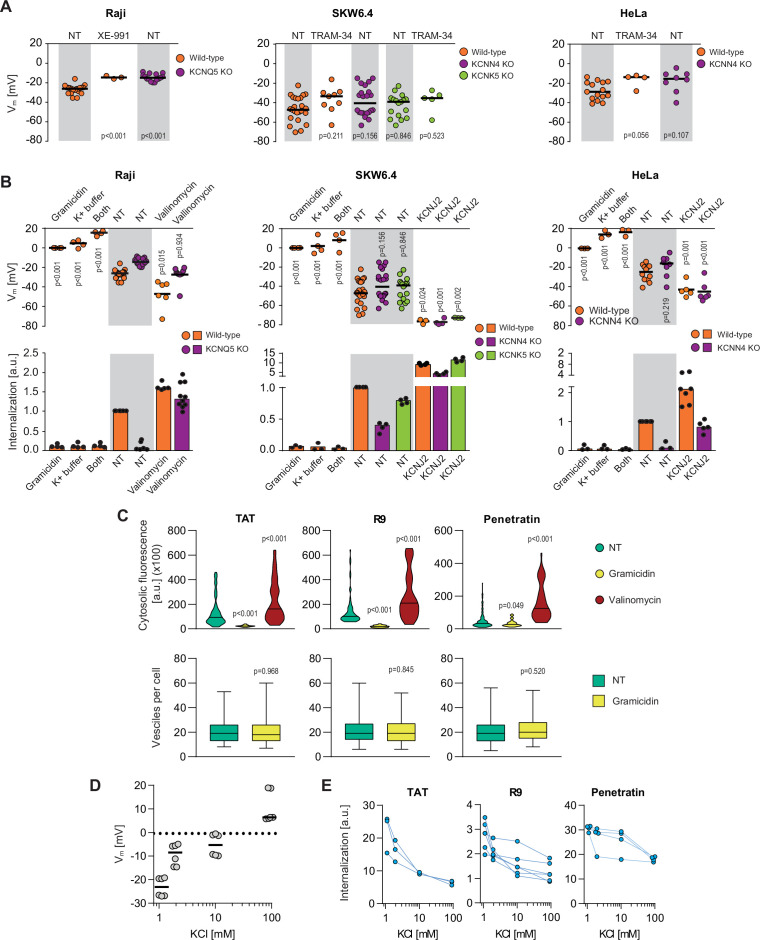
Figure 3
(A) Assessment of the resting plasma membrane potential in the indicated wild-type cell lines and the corresponding potassium channel knock-out (KO) clones in the presence or in the absence 10 μM XE-991 or TRAM-34. The gray and white zones correspond to non-treated cells and inhibitor-treated cells, respectively. NT, not treated. The p-values correspond to the assessment of the significance of the differences with the control wild-type condition using ANOVA multiple comparison analysis with Dunnett’s correction. Each dot in a given condition represents an independent experiment. (B) Effect of cellular depolarization (left of the gray zone) and hyperpolarization (right of the gray zone) on peptide internalization in the absence of serum. The indicated cell lines and the corresponding channel KO clones were pretreated or not with depolarization agents (2 μg/ml gramicidin for 5 min or high extracellular potassium buffer for 30 min) or with hyperpolarization inducer (10 μM valinomycin), followed by the addition of TAT-RasGAP317-326 for 1 hr. Alternatively, hyperpolarization was achieved by ectopic expression of the KCNJ2 potassium channel. Membrane potential and peptide internalization were then determined. Membrane potential was measured in the presence of DiBac4(3) by flow cytometry. Peptide internalization was measured by flow cytometry in the presence of 0.2% trypan blue. The p-values correspond to the assessment of the significance of the differences with the control wild-type condition using ANOVA multiple comparison analysis with Dunnett’s correction. Each dot in a given condition represents an independent experiment. Treatment with valinomycin was used in the absence of serum as the latter is expected to interfere with the drug (Rimmele and Chatton, 2014). As shown in Figure 3—figure supplement 5A, removing serum from the culture medium sensitized cells to TAT-RasGAP317-326 and consequently, the CPP concentration had to be adapted accordingly (Figure 3—figure supplement 5B). Serum withdrawal does not affect the Vm (Figure 3—figure supplement 5C). (C) Quantitation of cytosolic CPP signal (top) and the number of endocytic vesicles per cell (bottom) in wild-type HeLa cells (n = 158 cells) incubated for 1 hr with 10 μM FITC-CPP in control, depolarizing (2 μg/ml gramicidin), or hyperpolarizing (10 μM valinomycin) conditions in the absence of serum based on confocal microscopy images (Figure 3—figure supplement 2D). Comparison between different conditions to non-treated control was done using ANOVA test with Dunnett’s correction for multiple comparison. The number of endocytic vesicles per cell was quantitated based on confocal images. Statistical comparison was done using t-tests. Quantitation of vesicles was not performed in hyperpolarizing conditions due to masking from strong cytosolic signal. (D) Modulation of the Vm membrane potential by varying extracellular potassium concentrations. Assessment of membrane potential changes in Raji cells incubated in RPMI medium containing the indicated concentrations of potassium chloride (isotonicity was maintained by adapting the sodium chloride concentrations; see Materials and methods). Membrane potential was measured with DiBac4(3). The results correspond to the median of six independent experiments. (E) Internalization of various CPPs in the presence of different concentrations of potassium chloride in the media. Data for a given experiment are linked with thin blue lines.
Potassium channels maintain plasma membrane polarization that is required for cell-penetrating peptide (CPP) entry into cells.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Elife