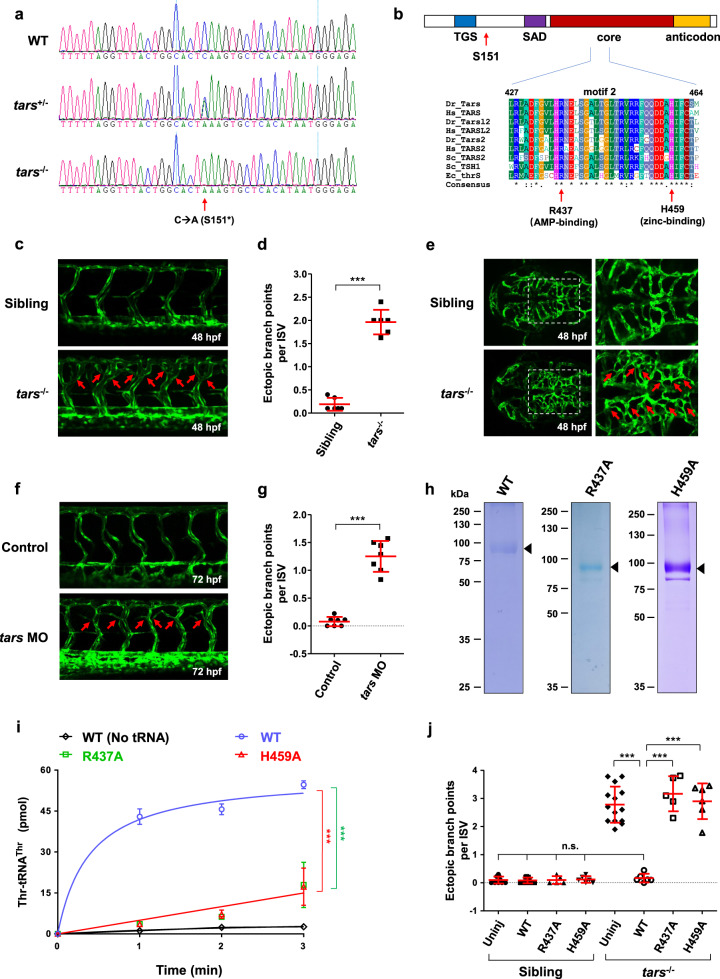
Fig. 1
a Sequencing results of the wild-type (WT) and the tars mutated heterozygous (tars+/−) and homozygous (tars−/−) zebrafish embryos. The arrow denotes the C-to-A nonsense mutation that generates a premature stop codon after serine 151 (S151*). b Domain architecture of the Tars protein, showing the position of serine 151 (S151; arrow), where the mutation-introduced stop codon predicts a deletion of the downstream SAD, core, and anticodon domains. Also shown is the alignment of the amino acid sequences of a signature motif (motif 2) within the core domain. Two evolutionarily conserved residues, R437 and H459, which are required for the aminoacylation activity and are directly involved in AMP and zinc binding, respectively, were chosen for functional studies. The alignment includes Tars and its homologs (i.e., Tars2 and Tarsl2, etc.) from humans (Hs, Homo sapiens), zebrafish (Dr, Danio rerio), yeast (Sc, Saccharomyces cerevisiae), and enterobacterial (Ec, Escherichia coli). c Confocal microscopy images of EGFP-labeled blood vessels in the trunk of the WT and tars−/− zebrafish embryos at 48 hpf. d Quantification and statistical analysis of the ectopic branch points per ISV of the tars−/− and sibling embryos. e An abnormal increase of branches in the hindbrain capillaries of the tars−/− embryos compared with the siblings. Magnified views of the dashed boxed regions are shown on the right. f Increased branch points in the ISVs caused by tars MO in WT embryos. In panels c, e, f, the arrows denote ectopic branch points of the vessels. g Quantification and statistical analysis of the ectopic branch points per ISV of the control and Tars knockdown embryos. h Coomassie blue staining of WT and mutant zebrafish Tars proteins, which were purified with His-tag from E. coli. i Aminoacylation activity assays with the purified proteins, showing the nearly abolished enzymatic activities of the R437A and H459A mutants compared with the WT Tars protein. j Rescue of the tars−/− angiogenic phenotype by injection of the WT, but not the inactivation mutant, tars mRNAs. Note that, for the tars−/− embryos, injection of the WT tars mRNA, but not the R437A or H459A mutant mRNA, significantly reduced the ectopic branch points per ISV compared with the uninjected tars−/− embryos (uninj). In contrast, for the WT embryos, injection of these WT, R437A, and H459A mRNAs showed no effect on the ISVs compared to the uninjected controls. Also, note that the phenotypic rescue by the WT tars mRNA was almost complete because quantification of the branch points of the injected embryos showed no difference compared with the WT embryos. In panels d, g, i, j, data are presented as means ± SD; two-tailed t-test; ***P < 0.001; n.s., not significant.