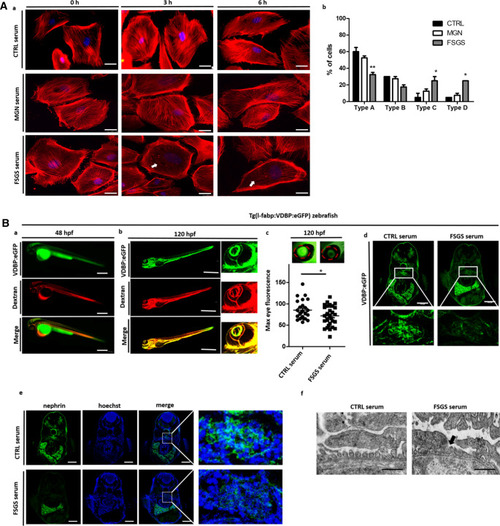
Cell culture and zebrafish assay to detect morphological and functional effects of unknown circulating permeability factors in FSGS. (A)—(a) Representative images of cultured differentiated human podocytes treated with 10% serum from a healthy control (CTRL serum), the patient with recurrent FSGS in the kidney transplant and a patient with membranous glomerulonephritis (incubation for 0 h, 3 h and 6 h). Cells were stained with phalloidin for cytoskeleton labeling. White arrows indicate cytoskeleton rearrangement. Scale bar = 25 µm. (b) Quantification of stress fiber formation in podocytes after treatment with different sera of patients. Type A: more than 90% of cell area filled with thick cables; type B: at least 2 thick cables running under nucleus and rest of cell area filled with fine cables; type C: no thick cables, but some cables present; type D: no cables visible in the central area of the cell. (B) Zebrafish assay for the detection of circulating permeability factors. (a) Representative image of a transgenic Tg(l-fabp:VDBP:eGFP) zebrafish larvae (VDBP:eGFP) injected with serum: dextran texas red into the zebrafish circulation (Dextran c.v. injection) at 48 hpf. Proper injection leads to red fluorescence of the zebrafish vascularization. Expression of the green fluorescent vitamin D binding protein just started. Scale bar = 500 µm. (b) At 120 hpf injected Tg(l-fabp:VDBP:eGFP) zebrafish express green fluorescent vitamin D binding protein (VDBP:eGFP) in the circulation. Red fluorescent serum: dextran mixture is still detectable in the circulation (dextran) and merges with the green fluorescent vitamin D binding protein (merge). The zebrafish eye is enlarged to show the retinal plexus. (c) Tg(l-fabp:VDBP:eGFP) transgenic zebrafish can be used to indirectly monitor proteinuria. Loss of green fluorescent protein in FSGS serum injected fish leads to reduced GFP signal in the retinal vessels where it can easily be quantified. Quantification of loss of fluorescent vitamin D binding proteins was done by measuring maximum GFP fluorescence in the retinal vessel plexus of Tg(l-fabp:VDBP:eGFP) zebrafish larvae at 120 hpf. Zebrafish larvae were injected with serum: dextran mixture from a healthy control and from a patient with FSGS recurrence in the kidney transplant at 48 hpf. *p < 0.05. n = 107. Scale bar = 500 µm. (d) Cryo sections of Tg(l-fabp:VDBP:eGFP) transgenic zebrafish larvae at 120 hpf showing systemic decrease in VDBP:eGFP in the systemic vascular system in FSGS serum injected zebrafish as a hint for proteinuria. Zebrafish were injected with serum from CTRL or FSGS patient at the time of disease recurrence. Scale bar = 100 µm. (e) Immunofluorescence staining for nephrin in FSGS and CTRL serum injected zebrafish at 120 hpf. Zebrafish larvae were injected with serum from a healthy control and from a patient with FSGS recurrence in the kidney transplant at 48 hpf. Scale bar = 100 µm. (f) Electron microscopy picture of the glomerular filtration barrier of 5 day old zebrafish larvae that were injected with either serum of the patient from the time of FSGS recurrence or with CTRL serum at 48 hpf. Black arrow head shows podocyte effacement. Scale bar = 500 nm. hpf: hours post fertilization, VDBP: Vitamin D binding protein.
|

