|
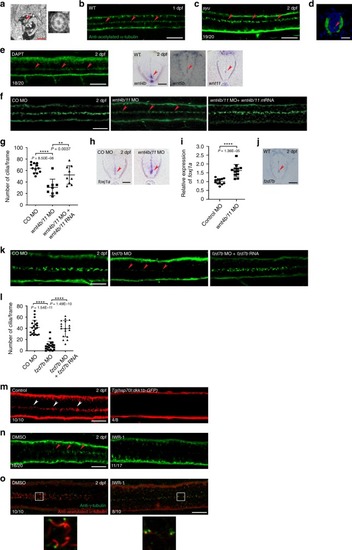
Wnt signaling is involved in the maintenance of ependymal motile cilia in zebrafish embryos.a Transmission electron microscopy (TEM) of the spinal cords (SCs) of zebrafish embryos at 2 dpf. Arrowhead indicates a motile cilium with the 9 + 2 microtubule configuration, which is magnified to the right. Scale bar = 1 μm. b Immunofluorescence (IF) staining of an embryo at 1 dpf with anti-acetylated-α-tubulin antibody. Dorsal view anterior to the left. Arrowheads represent motile cilia. Scale bar = 20 μm. c, d IF staining of sonic-you (syut4) mutant embryos at 2 dpf with anti-acetylated-α-tubulin antibody. Dorsal view anterior to the left (c). d The cross-section image of the SC ventral to the bottom. Arrowheads represent motile cilia. Scale bars = 20 μm. e Embryos were treated with DAPT (50 μM) at 34–48 hpf and IF stained with anti-acetylated-α-tubulin antibody. Dorsal view anterior to the left. Arrowheads represent motile cilia. Scale bar = 20 μm. f Embryos were co-microinjected with wnt4b MO and wnt11 MO (wnt4b/11 MO) alone or along with wnt4b mRNA and wnt11 mRNA (wnt4b/11 mRNA), and IF stained at 2 dpf with anti-acetylated-α-tubulin antibody. Arrowheads represent motile cilia. Dorsal view anterior to the left. Scale bar = 20 μm. CO: Control. g Quantification of the number of cilia per frame in embryos in f. Data are presented as mean ± SD. **P < 0.01 and ****P < 0.0001 by one-way ANOVA with Tukey’s honest significant difference (HSD) post hoc test (control morphants: n = 12 embryos; wnt4b/11 double morphants: n = 9 embryos; wnt4b/11 double morphants + wnt4b/11 mRNA: n = 9 embryos; one frame per embryo). h A cross-section images of the SCs of control morphants and wnt4b/11 double morphants at 2 dpf probed with foxj1a riboprobes ventral to the bottom. Arrowheads represent ECs. Scale bar = 20 μm. CO: Control. i RNAs were extracted from each group (20 embryos in h) at 2 dpf and levels of foxj1a mRNAs were assessed by qPCR. Mean ± SD. ****P < 0.0001 by two-tailed unpaired Student’s t test from four biological replicates (three technical replicates each). j A cross-section image of the SC of a WT embryo at 2 dpf probed with fzd7b riboprobes ventral to the bottom. Arrowhead represents ECs. Scale bar = 15 μm. k Embryos were microinjected with control MO, fzd7b MO or fzd7b MO + fzd7b mRNA, and IF stained at 2 dpf with anti-acetylated-α-tubulin antibody. Arrowheads represent motile cilia. Dorsal view anterior to the left. Scale bar = 20 μm. CO: Control. l Quantification of the number of cilia per frame in embryos in k. Mean ± SD. ****P < 0.0001 by one-way ANOVA with Tukey’s HSD post hoc test (control morphants: n = 21 embryos; fzd7b morphants: n = 21 embryos; fzd7b morphants + fzd7b mRNA: n = 18 embryos; one frame per embryo). mTg(hsp70l:dkk1b-GFP) embryos at 24 hpf were subjected to heat shock at 39 °C for 1 h, and IF stained at 2 dpf with anti-acetylated-α-tubulin antibody. Arrowheads represent motile cilia. Dorsal view anterior to the left. Scale bar = 20 μm. n, o Embryos were treated with IWR-1 (10 μM) at 8–48 hpf and IF stained with anti-acetylated-α-tubulin antibody only (n) or double immunostained with anti-acetylated-α-tubulin antibody and anti-γ-tubulin antibody (o) at 2 dpf. Arrowheads represent motile cilia. Dorsal view anterior to the left. The boxed areas in o are magnified in the respective lower panels. Scale bar = 20 μm.
|