Fig. S4
- ID
- ZDB-FIG-140626-27
- Publication
- Geurtzen et al., 2014 - Mature osteoblasts dedifferentiate in response to traumatic bone injury in the zebrafish fin and skull
- Other Figures
- All Figure Page
- Back to All Figure Page
|
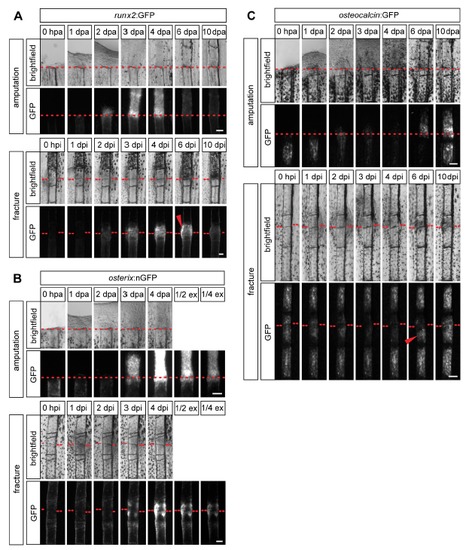
Timing of osteogenesis is similar in fractured versus amputated fin rays, but progression to late osteoblasts is delayed in fractures. (A) Time course of transgene expression in runx2:GFP fins during regeneration after amputation versus repair after fracture. GFP fluorescence is visible from 2 dpa/i in both injury models. runx2:GFP expression is reduced again to levels of uninjured fins at 6 dpa, while it is still high at this time point in fractured fin rays (arrowhead). (B) Time course of transgene expression in osterix:nGFP fins. GFP fluorescence is visible at 3 dpa/i in a similar intensity and is increased at 4 dpa/i in both injury models. ex, exposure. (C) Time course of transgene expression in osteocalcin:GFP fins. GFP fluorescence drops after injury up to 4 dpa/i in both injury models and recurs at 6 dpa in regenerating fins. In fractured fin rays expression levels are equally re-induced at 6 dpi in half of the cases looked at (arrowhead). GFP fluorescence throughout the fractured segment is completely re-established at 10 dpi. dpa, day(s) post amputation. dpi, day(s) post injury. |