Fig. 4
|
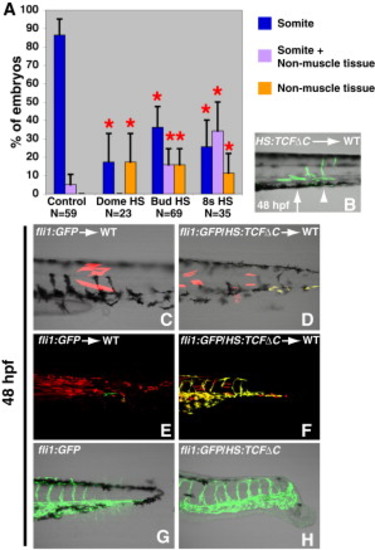
Wnt Signaling Specifies Paraxial Fate in Bipotential Paraxial/Endothelial Mesodermal Progenitors (A) In the absence of Wnt signaling, there is a significant shift in the resident location of cells from the somites to the nonmuscle mesoderm (note that the data for the somite clones is the same as that presented in Figure 3G, data for neural clones can be found in Figure 3). (B) An example of a single cell HS:TCFΔC transplant that was heat-shocked at the 8-somite stage, where several cells in the anterior of the clone have contributed normally to somites (arrow), whereas the cells in the posterior have contributed to a ventral mesoderm fate that appears to be vascular endothelium (arrowhead). (C and D) Rhodamine-labeled single cells were transplanted from the indicated donor and heat shocked at bud stage. (D) Wnt inhibition causes some of the progeny of the single cell to express the vascular marker fli1 (seen as yellow from the overlap of GFP and rhodamine). (E and F) Rhodamine-labeled cells were transplanted from the indicated donor and heat shocked at the 8-somite stage. (F) Wnt inhibition causes many of the transplanted cells to adopt a vascular fate instead of a muscle fate. (G and H) fli1:GFP and fli1:GFP/HS:TCFΔC embryos were heat shocked at the 8-somite stage. (H) Wnt inhibition causes a large increase in the amount of posterior vasculature. |
Reprinted from Developmental Cell, 22(1), Martin, B.L., and Kimelman, D., Canonical Wnt Signaling Dynamically Controls Multiple Stem Cell Fate Decisions during Vertebrate Body Formation, 223-232, Copyright (2012) with permission from Elsevier. Full text @ Dev. Cell