- Title
-
Bi-allelic variants in CELSR3 are implicated in central nervous system and urinary tract anomalies
- Authors
- Stegmann, J.D., Kalanithy, J.C., Dworschak, G.C., Ishorst, N., Mingardo, E., Lopes, F.M., Ho, Y.M., Grote, P., Lindenberg, T.T., Yilmaz, Ö., Channab, K., Seltzsam, S., Shril, S., Hildebrandt, F., Boschann, F., Heinen, A., Jolly, A., Myers, K., McBride, K., Bekheirnia, M.R., Bekheirnia, N., Scala, M., Morleo, M., Nigro, V., Torella, A., TUDP consortium, Pinelli, M., Capra, V., Accogli, A., Maitz, S., Spano, A., Olson, R.J., Klee, E.W., Lanpher, B.C., Jang, S.S., Chae, J.H., Steinbauer, P., Rieder, D., Janecke, A.R., Vodopiutz, J., Vogel, I., Blechingberg, J., Cohen, J.L., Riley, K., Klee, V., Walsh, L.E., Begemann, M., Elbracht, M., Eggermann, T., Stoppe, A., Stuurman, K., van Slegtenhorst, M., Barakat, T.S., Mulhern, M.S., Sands, T.T., Cytrynbaum, C., Weksberg, R., Isidori, F., Pippucci, T., Severi, G., Montanari, F., Kruer, M.C., Bakhtiari, S., Darvish, H., Reutter, H., Hagelueken, G., Geyer, M., Woolf, A.S., Posey, J.E., Lupski, J.R., Odermatt, B., Hilger, A.C.
- Source
- Full text @ NPJ Genom Med
|
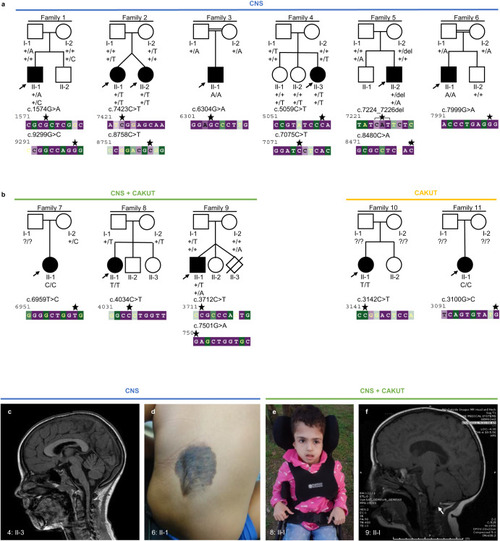
Families with bi-allelic variants in |
|
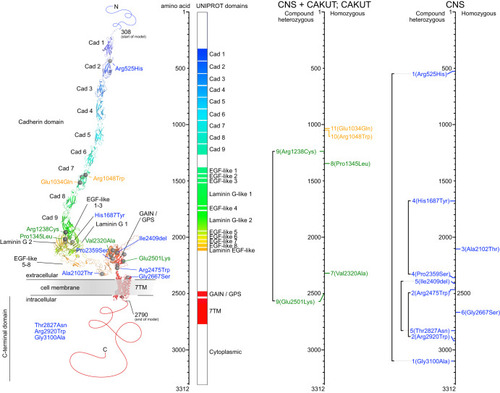
Structural modeling of CELSR3 and mapping of the variants. Structural modeling of CELSR3 and the respective variants according to the amino acid (aa) position. Left panel: 3D protein domain view and variant annotation using AlphaFold and PyMOL. Middle panel: Linearized aa view of the protein domains. Right panels: Variant location according to the respective phenotype categories: Central nervous system (CNS) anomalies in blue, combined CNS and congenital anomalies of the kidneys and urinary tract (CAKUT) in green, CAKUT only in yellow. Cad Cadherin, EGF Epidermal growth factor, GAIN G-protein-coupled receptor (GPCR) autoproteolysis-inducing domain, GPS GPCR proteolysis site, 7TM Seven-transmembrane. |
|
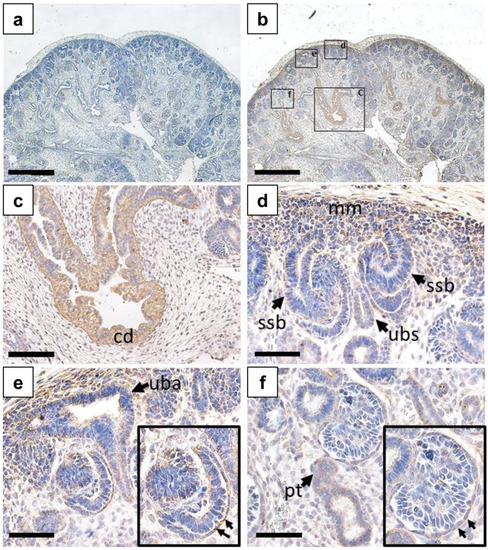
CELSR3 immunostaining in the human embryonic metanephric kidney at ten weeks gestation. All frames depict a ten-week gestation kidney with nuclei counterstained (blue) with hematoxylin. |
|
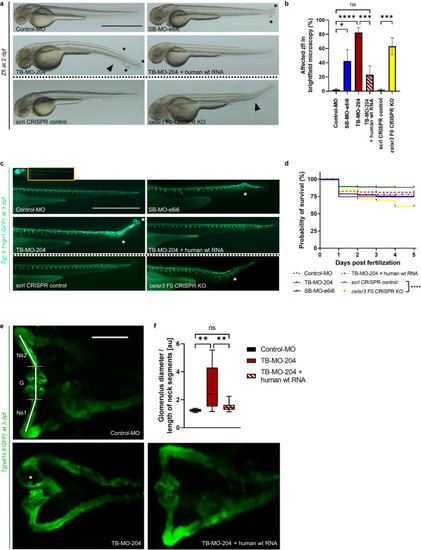
Transient suppression of Celsr3 in zebrafish larvae. Phenotypic evaluation of the different zebrafish larvae (zfl) groups: Zfl injected with Control-Morpholino (Control-MO), zfl injected with MO blocking EXPRESSION / LABELING:
PHENOTYPE:
|