- Title
-
Small leucine-rich proteoglycans inhibit CNS regeneration by modifying the structural and mechanical properties of the lesion environment
- Authors
- Kolb, J., Tsata, V., John, N., Kim, K., Möckel, C., Rosso, G., Kurbel, V., Parmar, A., Sharma, G., Karandasheva, K., Abuhattum, S., Lyraki, O., Beck, T., Müller, P., Schlüßler, R., Frischknecht, R., Wehner, A., Krombholz, N., Steigenberger, B., Beis, D., Takeoka, A., Blümcke, I., Möllmert, S., Singh, K., Guck, J., Kobow, K., Wehner, D.
- Source
- Full text @ Nat. Commun.
|
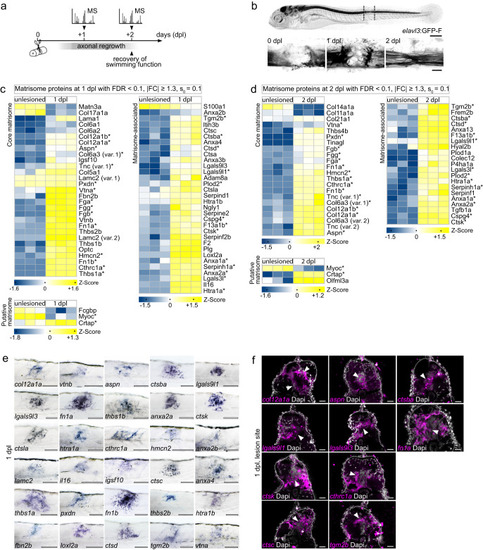
Mass spectrometry-based quantitative proteomics reveals changes in ECM composition during zebrafish spinal cord regeneration. |
|
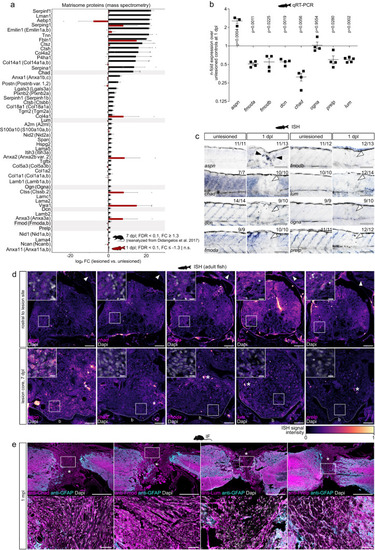
SLRPs are differentially enriched in CNS lesions of rodents and zebrafish. |
|
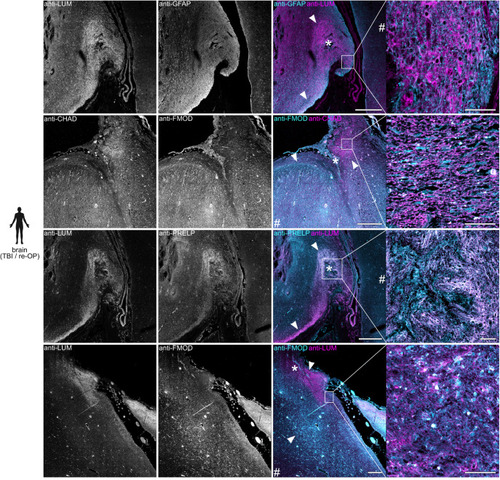
SLRPs are enriched in human brain lesions. Anti-CHAD, anti-FMOD, anti-LUM, and anti-PRELP immunoreactivity is locally increased in areas of scarring caused by contusion, local hemorrhage, or previous surgery (asterisks indicate lesion center, arrowheads mark scar boundaries) in the human brain, as compared to regions distant to the primary lesion site (hash sign), as well as human brain controls with no signs of fibrotic scarring (see Supplementary Fig. |
|
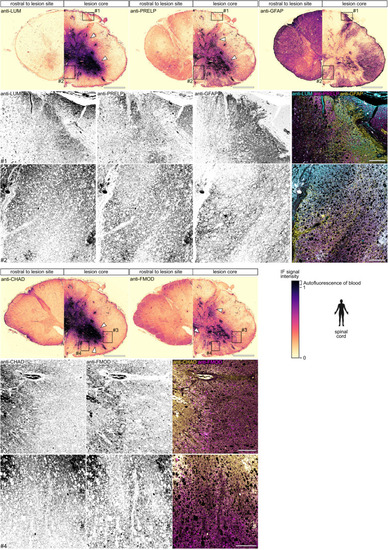
SLRPs are enriched in human spinal cord lesions. Anti-LUM, anti-PRELP, anti-CHAD, and anti-FMOD immunoreactivity is increased (arrowheads; see calibration bar of lookup table (LUT)) in the epicenter of the injured human spinal cord at nine days-post injury, as compared to rostral control segments of the same patient. Note that immunoreactivity is mainly observed in proximity to the hemorrhage (black; see calibration bar of LUT). Also note that with the exception of blood-derived autofluorescence, individual channels show distinct pattern of immunofluorescence (IF) signal (insets). Shown are transversal sections (dorsal is up). Similar results were obtained for anti-LUM in five out of six cases, for anti-PRELP and anti-FMOD in four out of six cases, and anti-CHAD in two out of six cases (Supplementary Table |
|
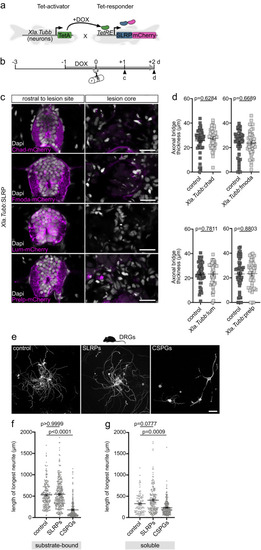
SLRPs are inhibitory to CNS axon regeneration. |
|
SLRPs do not directly act on neurons to inhibit axon regeneration. |
|
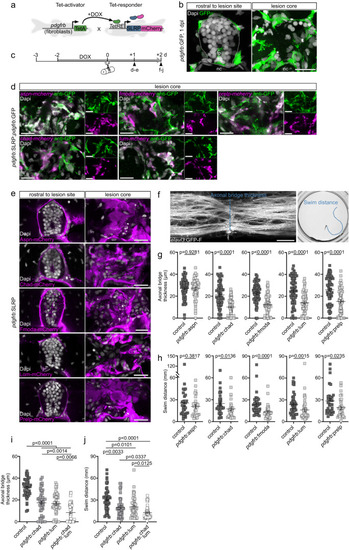
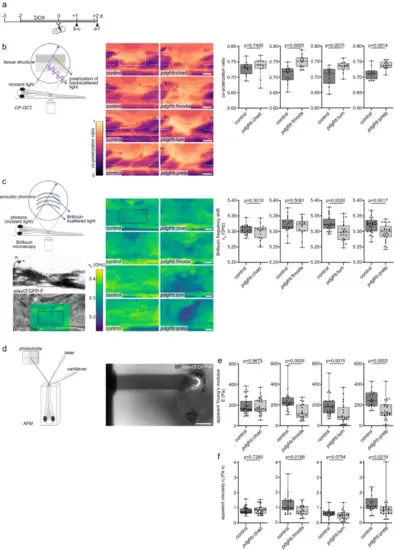
SLRPs modulate the structural and mechanical properties of the lesion environment a Timeline for experimental treatments shown in b–f. b Targeting Fmoda, Lum, or Prelp to the injury ECM in pdgfrb:SLRP transgenic zebrafish increases the co-polarization ratio in the spinal lesion site, as determined by cross-polarized optical coherence tomography (CP-OCT). Images shown are average intensity projections of the lesion site (lateral view; rostral is left). The dashed lines indicate the location of the severed spinal cord, the dashed rectangle indicates the region of quantification. Two-tailed Student’s t-test. ncontrol = 11, nChad = 11; ncontro = 12, nFmoda = 12; ncontro = 10, nLum = 12; ncontrol = 12, nPrelp = 12 animals. c Targeting Lum or Prelp to the injury ECM in pdgfrb:SLRP transgenic zebrafish decreases the Brillouin frequency shift ( ) in the spinal lesion site, as determined by Brillouin microscopy. Images shown are sagittal optical sections (brightfield intensity, confocal fluorescence, or Brillouin frequency shift map) through the center of the lesion site of an elavl3:GFP-F transgenic zebrafish (lateral view; rostral is left). The dashed rectangle indicates the region of quantification. Two-tailed Student’s t-test. ncontrol = 17, nChad = 17; ncontrol = 17, nFmoda = 16; ncontrol = 23, nLum = 22; ncontrol = 24, nPrelp = 24 animals over three (Chad, Fmoda) or four (Lum, Prelp) independent experiments. d–f Targeting Fmoda, Lum, or Prelp to the injury ECM in pdgfrb:SLRP transgenic zebrafish decreases the apparent Young’s modulus (E) (e; Fmoda, Lum, Prelp) and apparent viscosity (η) (f; Fmoda, Prelp) in the spinal lesion site, as determined by atomic force microscopy-based nanoindentation measurements (AFM). Recorded force-indentation curves were fitted to the Kelvin─Voigt─Maxwell model. Two-tailed Mann-Whitney test. ncontrol = 30, nChad = 26; ncontrol = 22, nFmoda = 17; ncontrol = 24, nLum = 23; ncontrol = 17, nPrelp = 23 animals over three (Chad, Fmoda, Prelp) or four (Lum) independent experiments. a–f Each data point represents one animal. Box plots show the median, first, and third quartile. Whiskers indicate the minimum and maximum values. Scale bars: 500 µm (d), 50 µm (b), 10 µm (c). BF brightfield intensity, BM Brillouin frequency shift map, d days, dpl days post-lesion, DOX doxycycline. Source data are provided as a Source Data file. |