- Title
-
Shifting the focus of zebrafish toward a model of the tumor microenvironment
- Authors
- Weiss, J.M., Lumaquin-Yin, D., Montal, E., Suresh, S., Leonhardt, C.S., White, R.M.
- Source
- Full text @ Elife
|
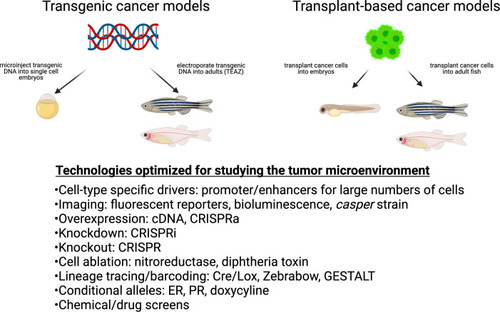
Cancer techniques in zebrafish.These include both transgenic and transplantation methodologies. While many of these techniques have been applied to the cancer cell itself, they are readily adaptable to the study of the microenvironment. |
|
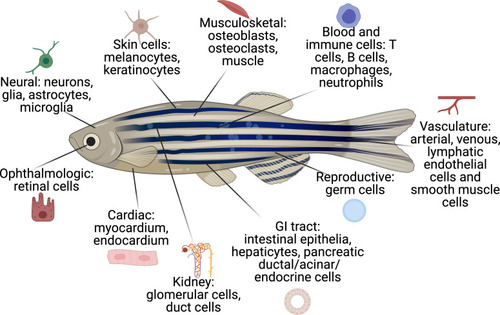
The cell types that have existing transgenic reporters in zebrafish.The specific promoter/enhancers driving expression in each of these cell types are shown in Supplementary file 1. |
|
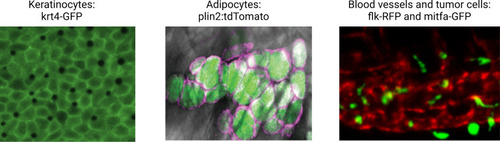
Illustrative examples of transgenic zebrafish reporters for specific cell types relevant to the microenvironment.Examples shown include the keratinocytes (marked by krt4-GFP), lipid droplets, and adipocytes (marked by a plin2:tdTomato fusion), and the blood vessel/melanoma interface (marked by the flk1-RFP and mitfa-GFP reporter, respectively). See Supplementary file 1 for a full list of references for these and other lines. Second panel is reproduced from Figure 1D in Lumaquin et al., 2021. |
|
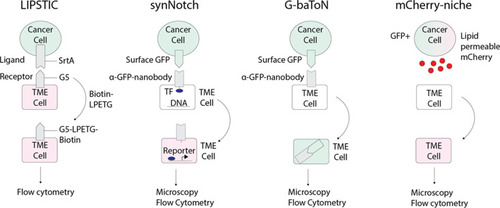
Emerging technologies that can be applied to track tumor/tumor microenvironment (TME) cell communication in zebrafish.LIPSTIC (Labeling Immune Partnerships by SorTagging Intercellular Contacts) utilizes the Staphylococcus aureus transpeptidase sortase A (SrtA) that can transfer peptides with LPXTG motifs to proteins containing five N-terminal glycine residues (G5) (Pasqual et al., 2018). By fusing SrtA to a ligand in a donor cell type and G5 to the cognate receptor of a receiver cell type, addition of LPETG marks the recipient G5-expressing cell. synNotch (synthetic Notch) is a versatile system in which a donor cell expresses a ligand or antigen, such as surface GFP, and the receiver cell expresses a chimeric receptor consisting of an extracellular sensing module such as an anti-GFP nanobody, a Notch core, and an intracellular transcription factor (Morsut et al., 2016). When the sensing module of the receiver cell is activated by interaction with the donor cell, the Notch core is cleaved leading to release of a transcription factor, such as Gal which can then activate downstream UAS reporters. G-baToN (GFP-based Touching Nexus) similarly utilizes surface GFP expression on a donor cell and anti-GFP nanobody expression on a receiver cell (Tang et al., 2020). Interaction of surface GFP with the anti-GFP nanobody leads to endocytosis and transfer of the GFP from the donor cell to the receiver cell, allowing for downstream analysis. mCherry-niche overexpresses a soluble and lipid permeable form of mCherry in donor cells, which is then acquired by neighboring receiver cells (Ombrato et al., 2019). By expressing mCherry-niche in GFP+ cancer cells, they can be distinguished from GFP - microenvironment cells that receive mCherry. |