- Title
-
An inducible expression system for the manipulation of autophagic flux in vivo
- Authors
- Schlotawa, L., Lopez, A., Sanchez-Elexpuru, G., Tyrkalska, S.D., Rubinsztein, D.C., Fleming, A.
- Source
- Full text @ Autophagy
|
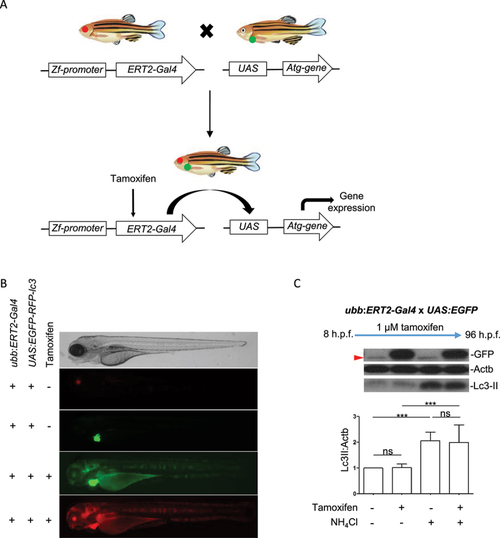
Regulation of transgene expression in |
|
Regulation of transgene expression using |
|
Expression of Atg5 increases autophagic flux in muscle cells. Eggs from a |
|
Temporal control of transgene expression to regulate autophagic flux. (a-c) Larvae from crosses of |
|
Autophagy upregulation by induction of Atg5 expression ameliorates pathology in a zebrafish model of tauopathy. (a) Schematic diagram of crosses to generate triple transgenic zebrafish. Photoreceptor degeneration is observed in the zebrafish transgenic line expressing the GFP-tagged human |