- Title
-
CRISPR-Cas9-mediated functional dissection of the foxc1 genomic region in zebrafish identifies critical conserved cis-regulatory elements
- Authors
- Ferre-Fernández, J.J., Muheisen, S., Thompson, S., Semina, E.V.
- Source
- Full text @ Hum. Genomics
|
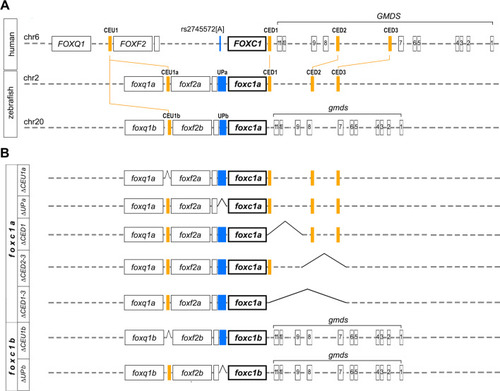
Schematic of human FOXC1, zebrafish foxc1a and foxc1b loci, and developed zebrafish lines. A Schematic drawing of human chromosome 6 aligned with zebrafish chromosomes 2 and 20 showing the positions of the identified conserved elements (filled orange boxes labeled at the top). Exons are indicated with labeled black boxes, while intergenic and intronic regions are shown with dotted lines. Position of rs2745572[A], an SNP associated with primary open-angle glaucoma, is shown in blue on human chromosome 6 and corresponding intergenic regions on zebrafish chromosomes 2 and 20 are marked with blue rectangles. B Schematic drawing showing generated lines and corresponding genomic deletions (sequence gaps are indicated) |
|
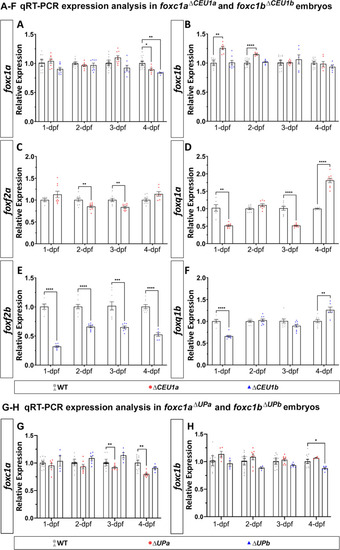
Changes in gene expression in mutants carrying deletions of upstream regions. A–F qRT-PCR relative expression of foxc1a (A) and foxc1b (B), foxf2a (C) and foxq1a (D) and foxf2b (E) and foxq1b (F) in 1-, 2-, 3- and 4-dpf wild-type, foxc1a∆CEU1a and/or foxc1b∆CEU1b homozygous zebrafish embryos (whole bodies). G, H qRT-PCR relative expression of foxc1a (G) and foxc1b (H) in 1-, 2-, 3- and 4-dpf wild-type, foxc1a∆UPa and foxc1b∆UPb homozygous embryos. β-actin (actb1) was used as the reference transcript in all experiments. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001 |
|
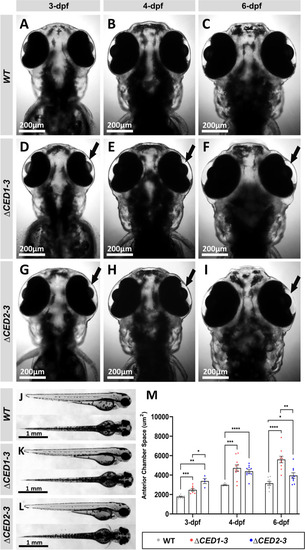
Phenotypic analysis of zebrafish mutants carrying deletions of downstream elements. A–I Dorsal images of the head region of 3-, 4- and 6-dpf wild-type (WT) (A–C), foxc1a∆CED1−3 (D–F) and foxc1a∆CED2−3 (G–I) homozygous zebrafish embryos. Both mutant lines showed the enlargement of the anterior chamber of the eye that was first noticeable at 3-dpf and became more pronounced by 6-dpf (black arrows in D–I). J–L Lateral and dorsal views of the 3-dpf wild-type (J), foxc1a∆CED1−3 (K) and foxc1a∆CED2−3 (L) homozygous zebrafish embryos. Please note no obvious morphological changes (aside from ocular defects presented in A–I) in mutant embryos. M Comparison of the anterior chamber area in wild-type and mutant embryos at 3-, 4-, and 6-dpf. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001 |
|
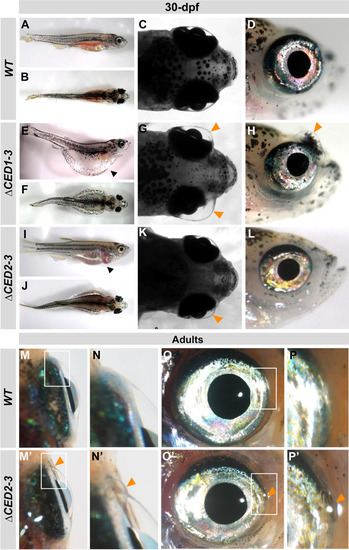
Developmental defects in juvenile and adult foxc1a∆CED1−3 and foxc1a∆CED2−3 mutants. A–L Lateral and dorsal whole body and head images of 30-dpf wild-type (A–D), foxc1a∆CED1−3 (E–H) and foxc1a∆CED2−3 (I–L) homozygous zebrafish embryos. Please note general swelling, including abdominal and heart edema (black arrowheads), in mutant embryos (E, I) as well as bilateral/unilateral enlargement of the anterior chamber of the eye, particularly in the dorso-nasal region (orange arrowheads in G–H, and K). M–P’ Ocular images of adult wild-type (M–P) and foxc1a∆CED2−3 mutants (M’–P’) showing bulging in the nasal part of the anterior chamber of the eye (orange arrowheads in N’–P’). Panels N, P, N’ and P’ show the regions outlined by white boxes in panels M, O, M’ and O’ at a higher magnification |
|
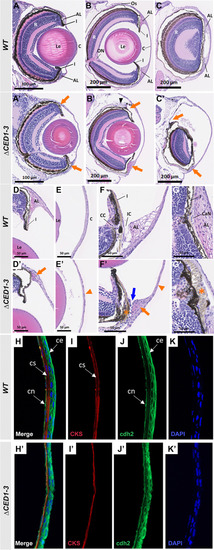
Histological analysis of ocular anomalies in foxc1a∆CED1−3 homozygous embryos. A, A’ H&E-stained transverse sections of the eye of 6-dpf wild-type and mutant embryos. B–C’ H&E-stained transverse sections through central (B and B’) and nasal (C and C’) eye regions of 30-dpf wild-type and mutant fish. Mutants show a marked enlargement of the anterior chamber and abnormal development of both dorsal and ventral annular ligaments (orange arrows, A’–C’); dislocation of lenses toward the back of the eye (B’); and deformed/misplaced scleral ossicles at the dorsal irido-corneal angle (black arrowhead, B’). D–E’ 20× magnifications of the dorsal irido-corneal angle (D and D’) and cornea (E and E’) showing details of the hypoplastic dorsal annular ligament (orange arrow, D’), and thin cornea at 30-dpf (orange arrowhead, E’). Transverse (F and F’) and coronal (G and G’) 40× magnifications of the ventral irido-corneal angle and canalicular network showing an apparent absence of the glycoprotein aggregates in the ventral annular ligament (orange arrow in F’), narrowing of the irido-corneal canal (blue arrow, F’), hyperplasia of the ventral iris stroma in this region (orange asterisks in F’ and G’) and thin cornea at 30-dpf (orange arrowhead in F’). H–K’ immunostaining of cornea sections of 30-dpf wild-type and mutant fish with anti-CKS (red) and anti-cdh2 (green), showing a thinner corneal stroma (I’) and a disorganized corneal epithelium (J’). AL, annular ligament; C, cornea; CaN, canalicular network; CC, ciliary canal; ce, corneal epithelium; cn, corneal endothelium; cs, corneal stroma; I, iris; IC, irido-corneal canal; Le, lens; ON, optic nerve; Os, scleral ossicle R, retina |
|
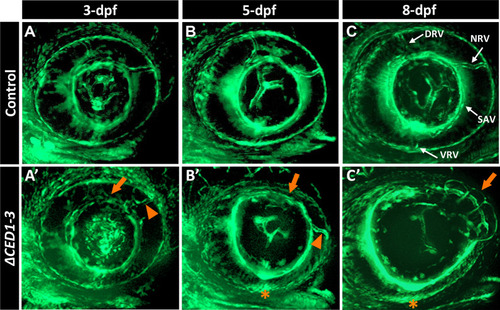
foxc1a∆CED1−3 mutant embryos display defects in the developing superficial choroidal vasculature. A–C’ Three-dimensional maximum intensity projection images of the ocular vasculature in live control (A–C) or foxc1a∆CED1−3 homozygous (A’–C’) embryos carrying fli1a:EGFP transgene at 3-, 5- and 8-dpf. Mutant embryos show abnormal development of the dorsal and nasal radial vessels (orange arrowheads in A’–C’), enlarged and deformed superficial annular vessel (orange arrows) and a highly disorganized vasculogenesis in the ventral part of the eye with no visible ventral radial vessel at 5- and 8-dpf (orange asterisks). DRV (dorsal radial), NRV (nasal radial), SAV (superficial annular), and VRV (ventral radial) blood vessels are indicated |
|
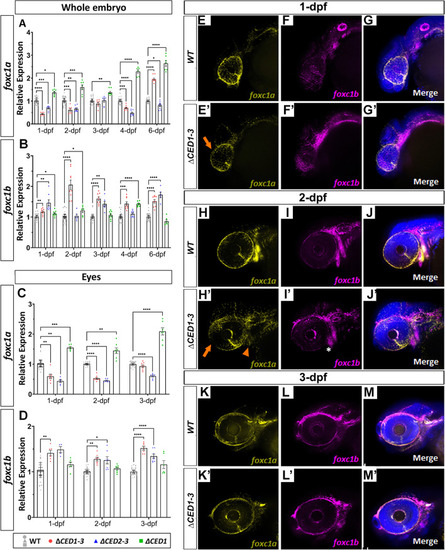
Expression studies in zebrafish mutants carrying deletions of downstream regions of foxc1a. qRT-PCR relative expression of foxc1a (A, C), foxc1b (B, D) transcripts in 1–6-dpf whole bodies (A, B) and 1–3-dpf dissected eyes (C, D) of wild-type and mutant embryos; *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001. E–M’ RNAscope in situ hybridization analysis of foxc1a (yellow) and foxc1b (magenta) expression in 1-, 2- and 3-dpf wild-type and foxc1a∆CED1−3 mutant embryos. Mutant embryos showed a visible reduction in ocular foxc1a expression at 1- and 2-dpf (orange arrows in E’ and H’) as well as in the branchial arches at 2-dpf (orange arrowhead in H’), while expression of foxc1b appeared normal (white asterisk; I’) |