- Title
-
NADPH-Oxidase Derived Hydrogen Peroxide and Irs2b Facilitate Re-oxygenation-Induced Catch-Up Growth in Zebrafish Embryo
- Authors
- Zasu, A., Hishima, F., Thauvin, M., Yoneyama, Y., Kitani, Y., Hakuno, F., Volovitch, M., Takahashi, S.I., Vriz, S., Rampon, C., Kamei, H.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
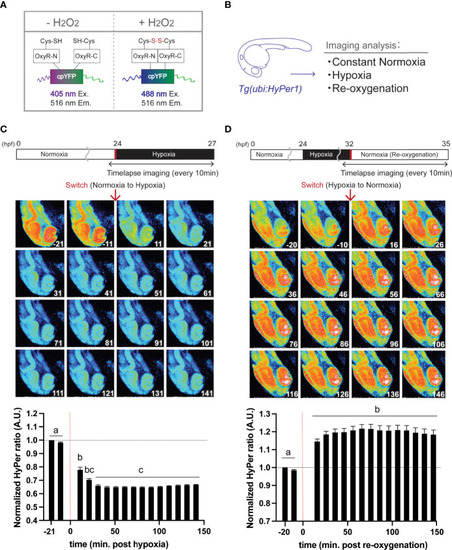
Hypoxia blunts H2O2 generation, but re-oxygenation regains it in HyPer1 transgenic zebrafish embryo. (A) Schematic illustration of fluorometric features of the HyPer1, a specific H2O2 sensor. (B) Experiment summary. The transgenic zebrafish embryos harboring ubiquitous HyPer1 expression were used. The embryos were placed in distinct environments (constant normoxia, hypoxia, and re-oxygenated normoxia) and subjected to imaging analysis. (C, D) Changes in H2O2 levels in zebrafish embryos during the transition from normoxia to hypoxia (C) or from hypoxia to normoxia (D). Transgenic fish expressing HyPer1 under the control of a ubiquitin gene promoter (ubi) were allowed to develop normally for up to 24 hr post-fertilization (hpf), and fluorescence signals were detected approx. 20 and 10 min before each treatment. After that, the rearing water of the embryos was replaced with hypoxic (C) or normoxic (D) water, and the images were timelapse photographed every 10 min for 2.5 hr after the transitions. The fluorescence intensity was analyzed in each image. The timing of the red arrows indicates the transition. The HyPer1 signal was calculated relative to the signal intensity of the first photo. Data are mean ± SE of 7 independent experiments. Values marked with different letters (a, b, c) are significantly different from each other (P<0.05), but values marked with common letters (b and bc; bc and c) are not significantly different from each other (P>0.05). |
|
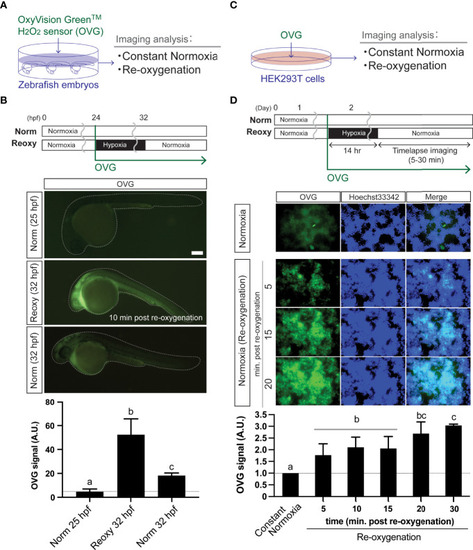
Re-oxygenation increases H2O2 generation in the zebrafish embryo and mammalian cells. (A) Experiment summary. The zebrafish embryos exposed with a specific H2O2 sensor probe (OxyVision Green™: OVG) were placed in distinct environments (constant normoxia: Norm and re-oxygenation: Reoxy) and subjected to imaging analysis. (B) The 24 hpf zebrafish embryos were exposed to hypoxia with 10 μM of OVG for 8 hr. Then, the fluorescence intensity at around 10 minutes after re-oxygenation was measured. The stage-matched (25 hpf Norm) and the chronological age-matched (32 hpf Norm) embryos were also tested for comparison. Bar, 100 μm. The OVG signal was calculated relative to the signal intensity of the 25 hpf Norm embryos. Data are mean ± SE of 5 independent experiments. The different letter denotes statistical significance at P<0.05. (C) Experiment summary. The human embryonic kidney cells (HEK293T cells) exposed with OVG were placed in Norm or Reoxy condition and subjected to imaging analysis. (D) The cells were exposed to 14 hr-long hypoxia with 5 μM of OVG. The OVG signal was calculated relative to the signal intensity of the Norm cells. Data are mean ± SE of 4-5 independent experiments. Values marked with different letters (a, b, c) are significantly different from each other (P<0.05), but values marked with common letters (b and bc; bc and c) are not significantly different from each other (P>0.05). |
|
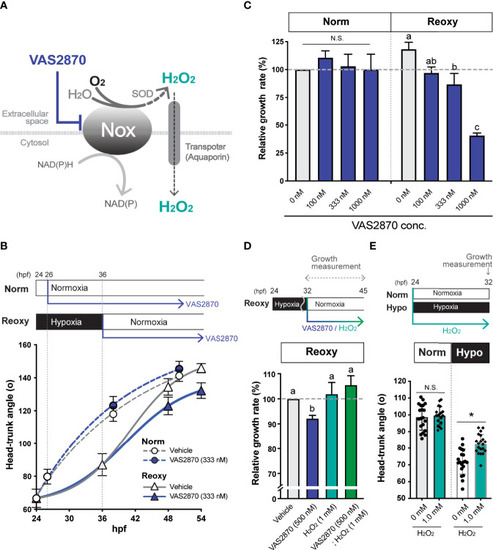
NADPH-oxidase (Nox)-generated H2O2 is crucial for the hypoxia/re-oxygenation-induced embryonic catch-up growth. (A) Schematic diagram of NADPH oxidase (Nox) function, which produces H2O2, a redox signaling molecule, near the plasma membrane. The Nox inhibitor, VAS2870, reduces the Nox-dependent production of H2O2 in the vicinity of the plasma membrane. Most Nox generate superoxide which is converted to the H2O2 by superoxide dismutase (SOD). (B, C) Effects of the Nox-inhibitor VAS2870. Wild-type zebrafish embryos were raised following the experimental regime depicted in the panel (B) diagram. Head-Trunk Angle (HTA) was determined at the indicated time points (B). Data are average ± SD, n=9-20. (C) Changes in the relative growth rate of embryos in the Norm (26-38 hpf) and Reoxy (36-48 hpf) groups. The control (vehicle=0.1% DMSO) group was set as 100%. Data are mean ± SE of 3 independent experiments. Values marked with different letters (a, b, c) are significantly different from each other (P<0.05), but values marked with common letters (a and ab; ab and b) are not significantly different from each other (P>0.05). N.S. means not significantly different (P>0.05). (D) Changes in the relative growth rate of embryos in the Reoxy (36-48 hpf) embryos treated with or without VAS2870 and H2O2. The vehicle alone group was set as 100%. Data are mean ± SE of 3 independent experiments. Values marked with different letters (a, b) are significantly different from each other (P<0.05). (E) Changes in HTA of the Norm and Hypo embryos at 32 hpf with or without 8 hr H2O2 treatment. Data are average ± SD, n=18-20. The asterisk (*) denotes statistical difference at P<0.05. N.S. means not significantly different (P>0.05). |
|
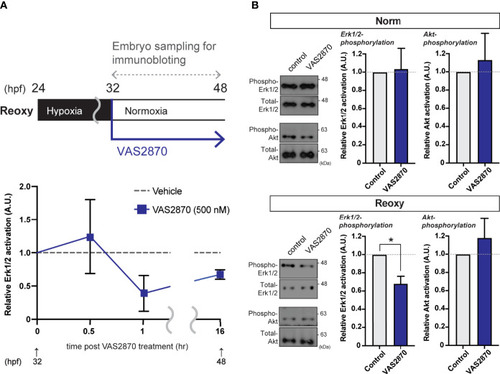
Nox-mediated generation of H2O2 is crucial for the hypoxia/re-oxygenation-induced Erk1/2-phosphorylation. (A) Time-course Erk1/2 phosphorylation changes of the VAS2870-treated embryos. Outline of experimental schedule is shown above the data. Reoxy embryos treated with or without VAS2870 were sampled at various timing, and the Erk1/2-phosphorylation levels were tested by immunoblotting. Relative Erk1/2-phosphorylation levels were shown as mean ± SE of 2-3 independent assays. (B) Erk1/2- and Akt- phosphorylation changes of the VAS2870-treated embryos. Embryos treated with or without VAS2870 (500 nM) were sampled at 48 hpf (16 hr post-re-oxygenation), and the Erk1/2- and Akt-phosphorylation levels were tested by immunoblotting. Relative Erk1/2- and Akt- phosphorylation levels were shown as mean ± SE of 2-3 independent assays. The asterisk (*) denotes statistical difference at P<0.05. |
|
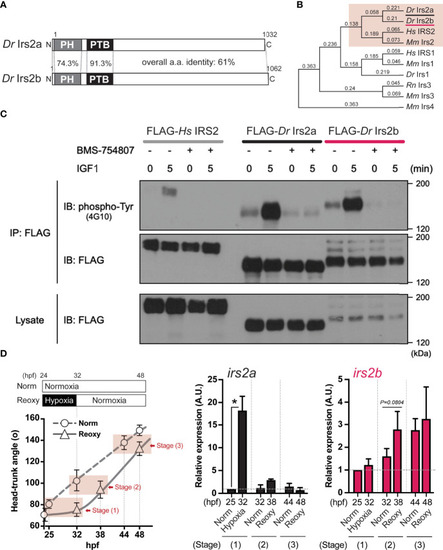
Identification, characterization, and expression of two zebrafish Irs2 genes (irs2a/b). (A) Schematic illustration of zebrafish Irs2 proteins (Dr Irs2a/2b). Characteristic pleckstrin homology (PH) domain and IRS-type phospho-tyrosine binding (PTB) domain are shown. (B) Phylogenetic analysis of the deduced amino acid sequence of Irs/IRS molecules based on the Neighbor joining method with bootstrap proportions. Dr, Danio rerio; Hs, Homo sapiens; Mm, Mus musculus; Rn, Rattus norvegicus. (C) Functional assay of zebrafish Irs2s in HEK293T cells. FLAG-tagged human IRS2 and zebrafish Irs2a/b were expressed, and the cells were treated with or without the specific IR/IGF-1R tyrosine kinase inhibitor BMS-754807 and were stimulated with IGF1 (100 ng/mL) for 5 minutes. Immunoprecipitation (IP) and immunoblot (IB) analyses were performed using denoted antibodies. (D) Real-time Q-PCR analysis of irs2a/b expression during zebrafish embryogenesis. Data are average ± SD, n=8-38. The sampling timing and the outline of experimental design are shown in the left. Embryos harboring statistically comparable body size was applied for the gene expression comparison: stage (1), 25 hpf Norm vs 32 hpf Hypo; stage (2) 32 hpf Norm vs 38 hpf Reoxy; stage (3), 44 hpf Norm vs 48 hpf Reoxy. Total RNA originating from whole embryos was used for cDNA synthesis, and the house-keeping gene (β-actin) expression was used for the internal control and normalization. The data are shown as ± SE of 3 independent assays. The Norm 25 hpf group is set as 1.0. The asterisk (*) denotes statistical difference at P<0.05. |
|
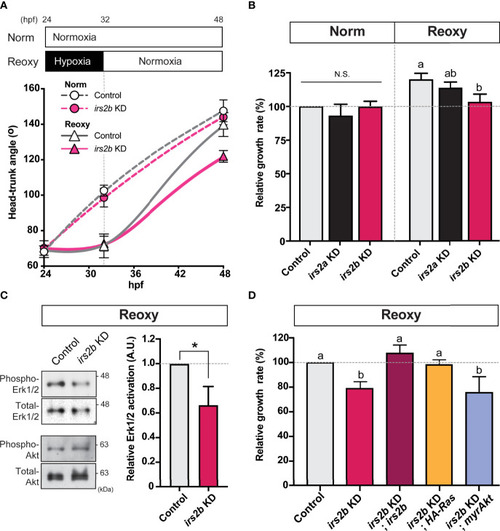
Irs2b, but not Irs2a, is required for Reoxy induced catch-up growth in the zebrafish embryo via its Reoxy-specific Erk1/2 activation function. (A) Changes in head-trunk angle. Data are average ± SD, n = 7-12. Embryos lacking the irs2b expression (irs2b KD) or control MO injected embryos were used for experiments. (B) Relative growth rates in Norm (26-32.5 hpf) and Reoxy (32-48.5 hpf) group. Data are mean ± SE of 3 independent experiments. Values marked with different letters (a, b) are significantly different from each other (P<0.05), but values marked with common letters (a and ab; ab and b) are not significantly different from each other (P>0.05). N.S. means not significantly different (P>0.05). (C) Immunoblot analysis of the phosphorylation levels of Akt and Erk1/2 under Reoxy condition (48 hpf). Data are mean ± SE of three independent experiments. The asterisk (*) denotes statistical difference at P<0.05. (D) Rescue experiments. Changes in head-trunk angle in the indicated experimental groups. Data are mean ± SE of 2-4 independent experiments. Values marked with different letters (a, b) are significantly different from each other (P<0.05). |
|
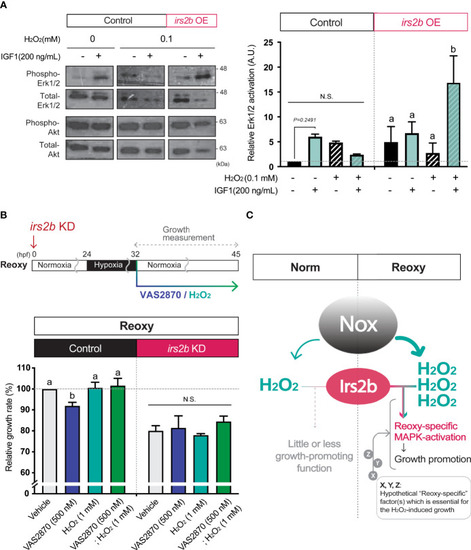
Irs2b mediates H2O2-dependent Erk1/2-phosphorylation and catch-up growth. (A) Immunoblot analysis of the IGF1 (200 ng/mL) -induced phosphorylation levels of Erk1/2 in the presence or absence of H2O2 and Irs2b in HEK293T cells. Cells harboring the irs2b overexpression (irs2b OE) or not (control) were used. Data are mean ± SE of 2 independent experiments. Values marked with different letters (a, b) are significantly different from each other (N.S. means not significantly different (P<0.05). (B) Changes in the relative growth rate of embryos in the Reoxy (32-45 hpf) embryos treated with or without VAS2870 and H2O2. Embryos lacking the irs2b expression (irs2b KD) or control MO injected embryos (Control) were used for experiments. The vehicle alone group was set as 100%. Data are mean ± SE of 3 independent experiments. Values marked with different letters (a, b) are significantly different from each other (P<0.05). (C) A proposed model. The Nox generates more H2O2 in Reoxy than in Norm, and the H2O2 facilitates Irs2b mediated Erk1/2 activation to induce catch-up growth. Thus the Irs2b serves as a downstream effector of re-oxygenation-induced H2O2. Since the excess H2O2 is insufficient for the significant growth acceleration in the Norm condition, other hypothetical factors (X, Y, Z, in this model) collaborating with the H2O2-Irs2b-Erk1/2 signaling would be involved in this catch-up growth model. |