- Title
-
Scrutinizing pathogenicity of the USH2A c.2276 G > T; p.(Cys759Phe) variant
- Authors
- Reurink, J., de Vrieze, E., Li, C.H.Z., van Berkel, E., Broekman, S., Aben, M., Peters, T., Oostrik, J., Neveling, K., Venselaar, H., Ramos, M.G., Gilissen, C., Astuti, G.D.N., Galbany, J.C., van Lith-Verhoeven, J.J.C., Ockeloen, C.W., Haer-Wigman, L., Hoyng, C.B., Cremers, F.P.M., Kremer, H., Roosing, S., van Wijk, E.
- Source
- Full text @ NPJ Genom Med
|
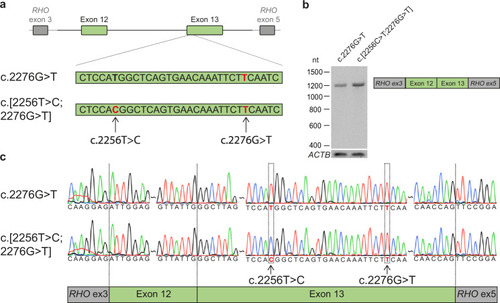
Minigene splice assays for variants c.2256 C > T and c.2276 G > T.
a A minigene splice assay was performed with a construct spanning from USH2A intron 11 to intron 13 (6,814 nt), containing either c.2276 G > T or both variants (c.[2256 T > C;2276 G > T]). b A single RT-PCR product of 1112 nt was observed after expression of both splice vectors, indicative of the incorporation of USH2A exons 12 and 13 between RHO exons 3 and 5 in both transcripts. c Sanger sequencing confirmed that USH2A exon 12 and exon 13 were correctly incorporated in the mRNA. |
|
Generation of the usherinp.(Cys771Phe) zebrafish model.
a Eggs were injected at a one-cell stage with CRISPR/Cas9 mix containing both the target specific single guide RNA (sgRNA) and the 126 nt homology directed repair (HDR) template (partially depicted, in blue) for incorporation of the c.2312 G > T (p.(Cys771Phe)) variant (in red) and protospacer-adjacent motif (PAM, in orange) disrupting variant (c.2304 C > T, in green). The sgRNA target region is depicted in purple. b Once zebrafish were at reproductive age, eggs of the initially injected zebrafish were screened for transmission of the c.2312 G > T and PAM disturbing variant. Three out of ten zebrafish that were screened transmitted the c.2312 G > T variant to their offspring. c Crossbreeding of a c.2312 G > T (p.(Cys771Phe)) zebrafish with a wildtype zebrafish was performed for two generations to reduce unforeseen off-target effects. After the first crossbreeding, genomic DNA was screened for predicted off-target effects of our CRISPR-Cas9 strategy and RNA of homozygous larvae was screened from exon 12 to 14 for deviations on transcript level. d Two p.(Cys771Phe) zebrafish were crossbred with each other to produce homozygous zebrafish. The phenotype of five-day-old larvae was then investigated with immunohistochemistry and electroretinography. |
|
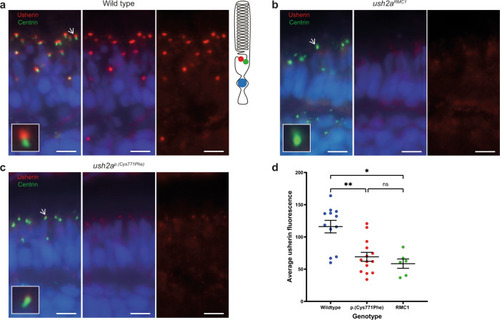
Reduced expression level of usherinp.(Cys771Phe) at the photoreceptor periciliary membrane.
a In wildtype zebrafish larval eyes (n = 12 eyes), usherin (red signal) localizes at the photoreceptor periciliary membrane adjacent to the basal body and connecting cilium marker centrin (green signal) as shown by the schematic representation of a photoreceptor on the right. A magnification of one photoreceptor (indicated by an arrow) is depicted in the inlay. b In ush2armc1 knock-out larvae usherin is not detectable (n = 6 eyes). c Localization of usherin at the photoreceptor periciliary membrane was strongly reduced in eyes of ush2ap.(Cys771Phe) larvae (n = 14 eyes) as compared to wildtype. d A Kruskal–Wallis test was performed based on the average of the mean grey value for usherin adjacent to each centrin spot and confirmed a significant decrease of usherin localization adjacent to centrin for the usherinp.(Cys771Phe) and the usherinRMC1 models. The average grey value per retinal section was plotted in a scatter plot (mean ± SEM). Nuclei are stained with DAPI (blue signal). Scale bar: 5 µM. **p: 0.0094, *p: 0.0107, ns not significant. |
|
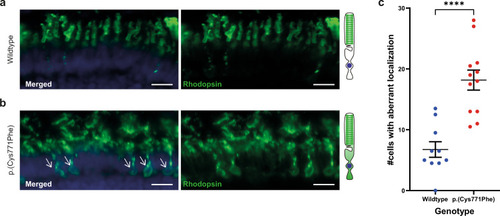
Rhodopsin localization to the photoreceptor cell body in the ush2ap.(Cys771Phe) zebrafish.
a Rhodopsin (green signal) localizes to the rod photoreceptor outer segments in wildtype larval eyes. A visual representation of a photoreceptor is shown on the right. b Aberrant localization of rhodopsin to the photoreceptor cell body was observed in ush2ap.(Cys771Phe) larvae (indicated by white arrows), which was observed in a significantly higher number of photoreceptors of ush2ap.(Cys771Phe) larvae as compared to wildtype larvae (c; unpaired t test). Nuclei are stained with DAPI (blue signal). Scale bar: 10 µM. ****p < 0.0001. |
|
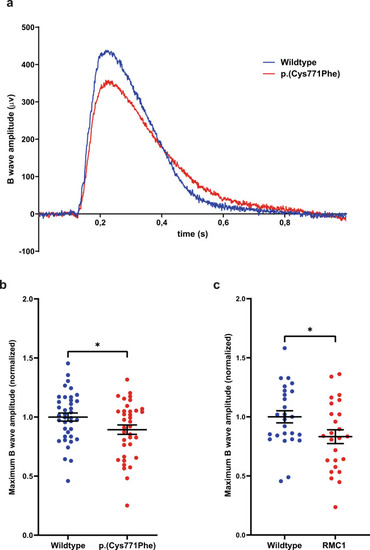
Electroretinogram recordings show that ush2ap.(Cys771Phe) mutants are vision impaired.
a Representative electroretinograms of a p.(Cys771Phe) zebrafish and a wildtype sibling at 5 days post-fertilization. b The maximum B wave amplitude was significantly lower in p.(Cys771Phe) zebrafish as compared to wildtype siblings (unpaired t test). The average wildtype amplitude was normalized to 1. Each datapoint corresponds to recordings from an individual larvae (mean ± SEM). *p: 0.0445. c A comparative analysis of ush2armc1 knock-out larvae and age- and strain-matched wildtype larvae was shown to result in a similar and significant decrease in maximum B wave amplitude. Again, the average wildtype amplitude was normalized to 1. Each datapoint corresponds to recordings from an individual larvae (unpaired t test, mean ± SEM). *p: 0.0345. PHENOTYPE:
|