- Title
-
Glyoxylate protects against cyanide toxicity through metabolic modulation
- Authors
- Nielson, J.R., Nath, A.K., Doane, K.P., Shi, X., Lee, J., Tippetts, E.G., Saha, K., Morningstar, J., Hicks, K.G., Chan, A., Zhao, Y., Kelly, A., Hendry-Hofer, T.B., Witeof, A., Sips, P.Y., Mahon, S., Bebarta, V.S., Davisson, V.J., Boss, G.R., Rutter, J., MacRae, C.A., Brenner, M., Gerszten, R.E., Peterson, R.T.
- Source
- Full text @ Sci. Rep.
|
Glyoxylate identified as cyanide antidote in metabolite screen. ( |
|
Glyoxylate improves survival rates in vertebrate animal models. ( |
|
Oxygen metabolism is restored following glyoxylate treatment. ( |
|
Glyoxylate’s rescue mechanism is not fully explained by cyanohydrin formation. Dose dependent survival of 5 dpf zebrafish in the presence of ( |
|
Direct metabolites of glyoxylate do not rescue with comparable efficacy. ( |
|
LDH is crucial for glyoxylate metabolism. ( |
|
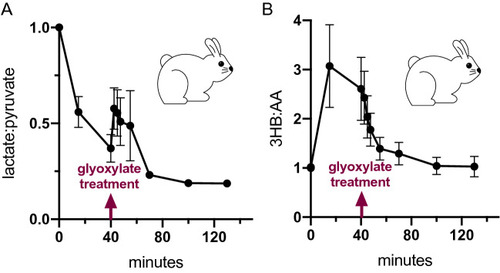
Glyoxylate reverses compartmentalized redox imbalance. ( |