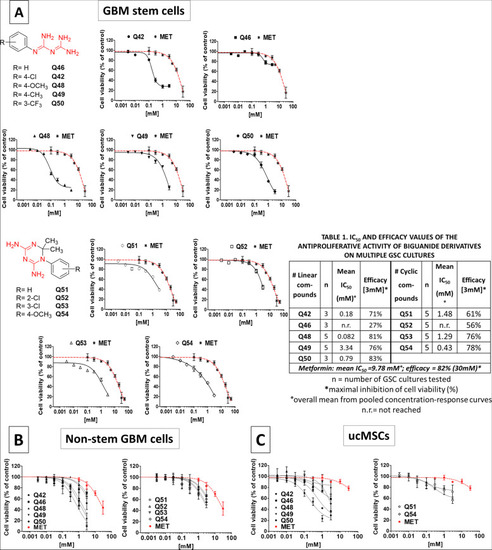
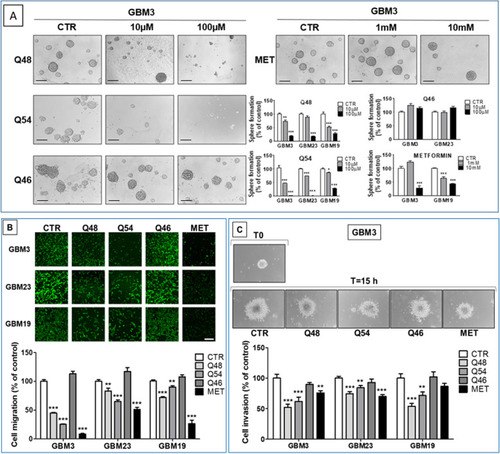
- Title
-
Chloride intracellular channel 1 activity is not required for glioblastoma development but its inhibition dictates glioma stem cell responsivity to novel biguanide derivatives
- Authors
- Barbieri, F., Bosio, A.G., Pattarozzi, A., Tonelli, M., Bajetto, A., Verduci, I., Cianci, F., Cannavale, G., Palloni, L.M.G., Francesconi, V., Thellung, S., Fiaschi, P., Mazzetti, S., Schenone, S., Balboni, B., Girotto, S., Malatesta, P., Daga, A., Zona, G., Mazzanti, M., Florio, T.
- Source
- Full text @ J. Exp. Clin. Cancer Res.
|
|
|
|
|
|
|
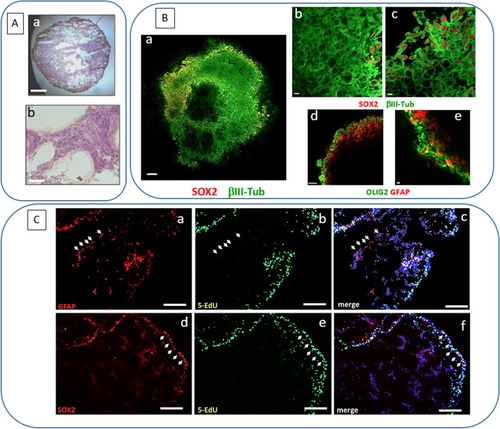
Characterization of GSC 3D organoids. |
|
|
|
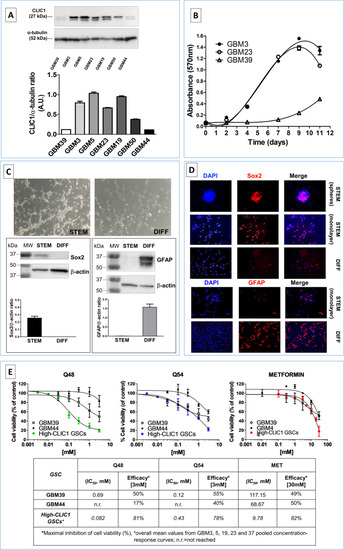
Characterization of GSC cultures expressing low levels of CLIC1 protein. |
|
|