- Title
-
Plasma obtained following murine hindlimb ischemic conditioning protects against oxidative stress in zebrafish models through activation of nrf2a and downregulation of duox
- Authors
- Guan, R., Wen, X.Y., Leung, C.H., Ciano-Oliveira, C.D., Lam, S., Dai, S.Y., Karbassi, F., Mauro, A., Wang, Y., Rotstein, O.
- Source
- Full text @ PLoS One
|
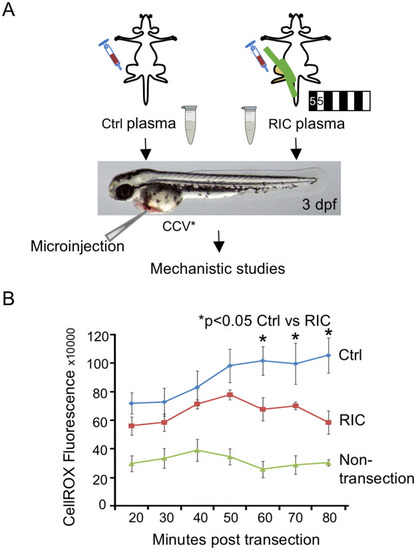
RIC plasma reduces ROS generation in the tail wound in zebrafish.
(A)Plasma from mice subjected to RIC (4 cycles of 5-min hind limb ischemia/reperfusion) or control (Ctrl) was microinjected into common cardinal vein of Tg (mpx:EGFP) zebrafish at 3 day post fertilization. (B) The graph shows the CellROX fluorescence intensity detected at different time points in RIC, Ctrl and non-tailfin transection groups. *p<0.05. |
|
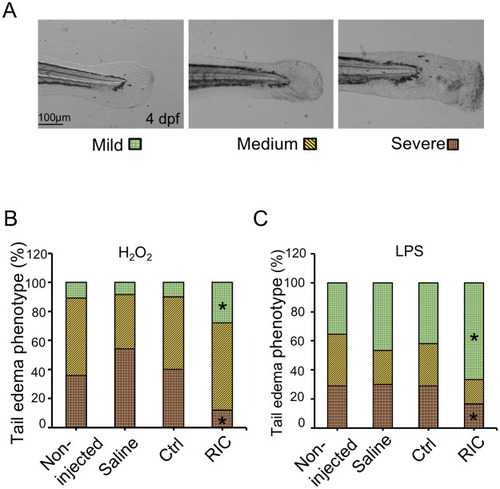
RIC protects against oxidative stress induced by H2O2 or LPS.
(A) H2O2 or LPS induces tail fin edema in zebrafish larvae. The phenotype of the tailfin edema was categorized into three groups according to the extent of damage: mild, medium, and severe. (B) Zebrafish injected with saline, Ctrl or RIC Plasma were subjected to H2O2 (25mM) for 1.5 hours. Percentage of each phenotype for each group were shown in graph. *P < 0.05. (C) zebrafish injected with saline, Ctrl or RIC Plasma were subjected to LPS (100 μg/mL) for 6 hours. Percentage of each phenotype for each group were shown in graph. *P < 0.05. |
|
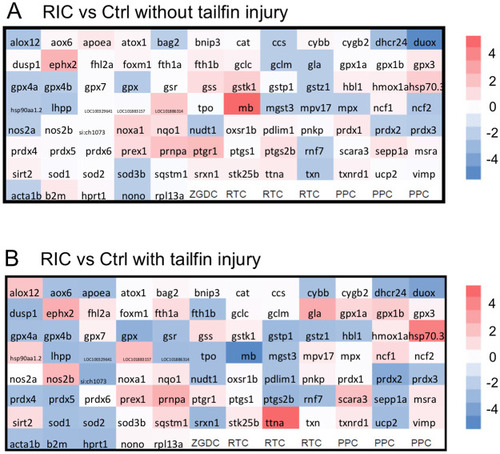
Real-time PCR array revealed that oxidative stress related genes are affected in RIC treated fish.
(A) Zebrafish larvae injected with Ctrl or RIC plasma were incubated at 28°C for the same amount of time as the tailfin cut group was harvested. The fish larvae without tailfin injury were collected for RNA extraction and PCR array analysis. The relative gene expression (fold change, RIC group/ Ctrl group) is shown in the heatmap. The up-regulated genes in RIC treated fish are represented in red (fold>1), while the down-regulated gene are represented in blue (fold<-1). (B) Zebrafish larvae injected with Ctrl or RIC plasma were incubated at 28°C for 16 hours. The tail fin was transected and incubated for 1 hour. The fish larvae with tailfin injury were collected for RNA extraction and PCR array analysis. |
|
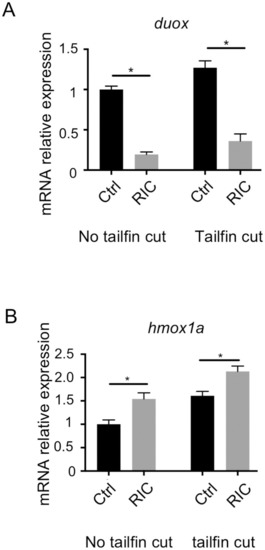
The downregulation of duox and upregulation of hmox1a are confirmed in RIC-treated fish.
(A) Real-time PCR data confirmed that the expression of duox at mRNA level is significantly down-regulated in RIC treated fish (p<0.05). (B) Real-time PCR analysis confirmed that the expression of hmox1a at mRNA level is significantly up-regulated in RIC treated fish (p<0.05). |
|
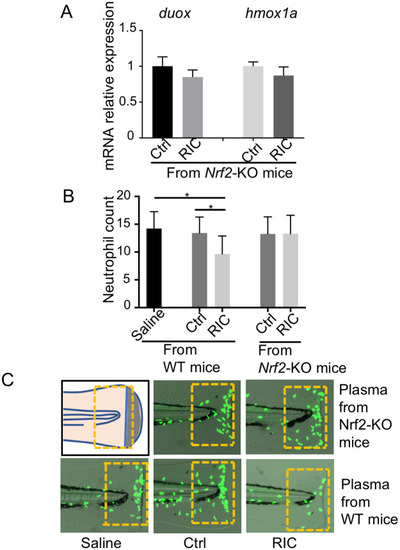
nrf2a plays a critical role on the protective effect of RIC.
(A) Real time PCR showed the expression level of duox and hmox1a in the fish treated with the plasma derived from Nrf2-KO mice. (B) RIC plasma derived from Nrf2 KO animals did not exert inhibiting effect on neutrophil migration. (n = 28) *p<0.05. (C) Representative images of neutrophil migration from each group were shown. Yellow rectangular frames were used for quantitation. |
|
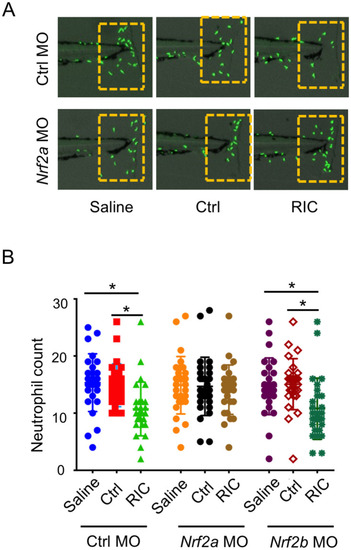
Knockdown of nrf2a abolished inhibiting effect of RIC on neutrophil migration.
(A) RIC plasma shows significant inhibiting effect on neutrophil migration to the site of tailfin injury in MO-Ctrl and MO-nrf2b injected larvae, *p<0.05. However, there was no significant difference between these group in MO-nrf2a injected larvae. Representative images of neutrophils in the tailfin wound area were shown for each group. (B) Graph bars display knockdown of nrf2a by MO-nrf2a abolished inhibiting effect of RIC on neutrophil migration to the tail wound. |
|
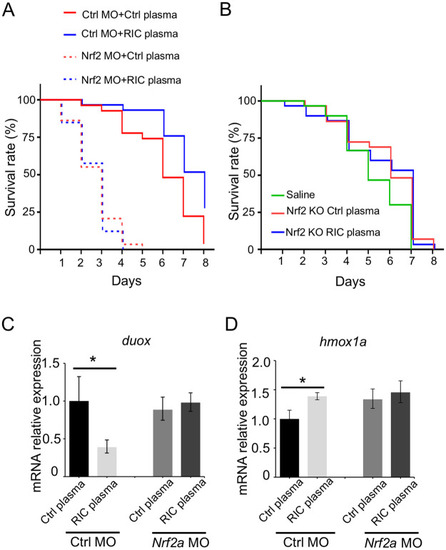
RIC works through activation of nrf2a in zebrafish.
(A) Kaplan-Meier survival curve of zebrafish subjected to LPS treatment. nrf2a knockdown resulted in earlier mortality in response to LPS exposure (80 μg/mL) and the protective effect of RIC Plasma was abolished in nrf2a knockdown zebrafish (n = 26–33). (B) RIC Plasma from Nrf2 KO mice did not exert survival benefit in response to LPS exposure (80 μg/mL) induced mortality in zebrafish (n = 26–30). (C) Real time PCR showed the expression level of duox in the fish treated with the plasma in nrf2a-knockdown fish. *p<0.05. (D) Real time PCR showed the expression level of hmox1a in the fish treated with the plasma in nrf2a-knockdown fish. *p<0.05. |
|
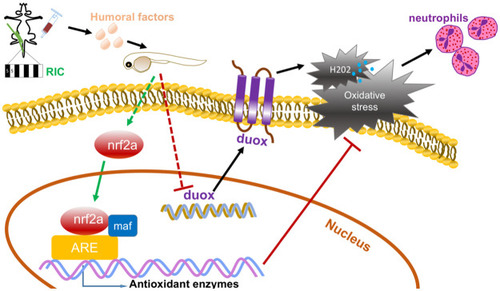
Graphical illustration of the protective effect of RIC in zebrafish.
|