- Title
-
Inducible Liver Cancer Models in Transgenic Zebrafish to Investigate Cancer Biology
- Authors
- Lee, A.Q., Li, Y., Gong, Z.
- Source
- Full text @ Cancers
|
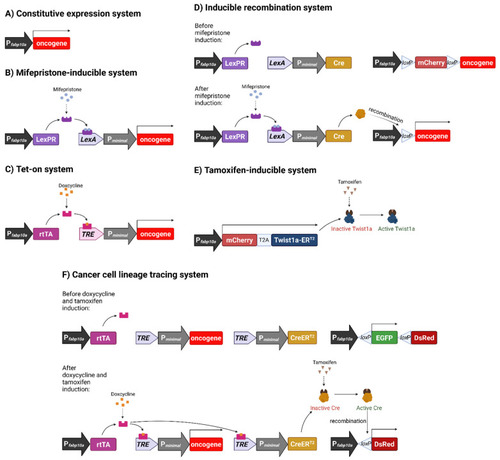
Schematic representation of various inducible systems we used for development of liver cancer models in transgenic zebrafish. (A) Constitutive expression system. Oncogene expression is under direct control of the hepatocyte-specific fabp10a promoter, causing oncogene to be constitutively expressed. (B) Mifepristone-inducible system. fabp10a promoter is used to transcribe a chimeric LexPR transactivator, which consists of the DNA binding domain of the bacterial LexA protein, the ligand binding domain of the human progesterone receptor (PR) and the activation domain of human p65 transcription factor. LexPR transactivator is activated by the mifepristone ligand and binds to the LexA operator to activate the transcription of the downstream oncogene. (C) Tet-on system. The reverse tetracycline-controlled transactivator (rtTA) is transcribed by the fabp10a promoter, but it becomes activated only in the presence of doxycycline and, in turn, it binds to the Tet Response Element (TRE) to activate the transcription of downstream oncogene. (D) Inducible recombination system. It incorporates the Cre-loxP components into the mifepristone-inducible system in order to induce permanent oncogene expression via genomic recombination. In this system, mifepristone activates LexPR, which binds the LexA operator to allow the expression of Cre recombinase. Cre recognises loxP sequences and excises the mCherry STOP cassette to permanently activate the downstream oncogene. (E) Tamoxifen-inducible system. A Twist1a-ERT2 fusion gene (twist1a and ligand binding domain of estrogen receptor [ER]) is constitutively transcribed by fabp10a promoter but the activation of the fusion protein requires the ligand, tamoxifen. T2A is a self-cleavage peptide to allow simultaneous production of two separate proteins. (F) Cancer cell lineage tracing system. Doxycycline activates rtTA, which in turn causes transcription of both xmrk oncogene and CreERT2. CreERT2 is activated by tamoxifen and excises the floxed EGFP sequence to cause the change of the expression of fluorescent protein genes. Figure created with BioRender.com (accessed on 23 September 2021). |
|
Summary of key pathways and mechanisms involved in tumour development and regression in our transgenic zebrafish models of liver cancer. The figure displays the main liver cells (hepatocytes and cholangiocytes) and immune cell types, cytokines and hormones that contribute to or exacerbate tumorigenesis and progression to liver cancer, and the crosstalk between them. The three mechanisms for tumour regression are also shown. Figure created with BioRender.com (accessed on 17 August 2021). |