- Title
-
Approved drugs ezetimibe and disulfiram enhance mitochondrial Ca2+ uptake and suppress cardiac arrhythmogenesis
- Authors
- Sander, P., Feng, M., Schweitzer, M.K., Wilting, F., Gutenthaler, S.M., Arduino, D.M., Fischbach, S., Dreizehnter, L., Moretti, A., Gudermann, T., Perocchi, F., Schredelseker, J.
- Source
- Full text @ Br. J. Pharmacol.
|
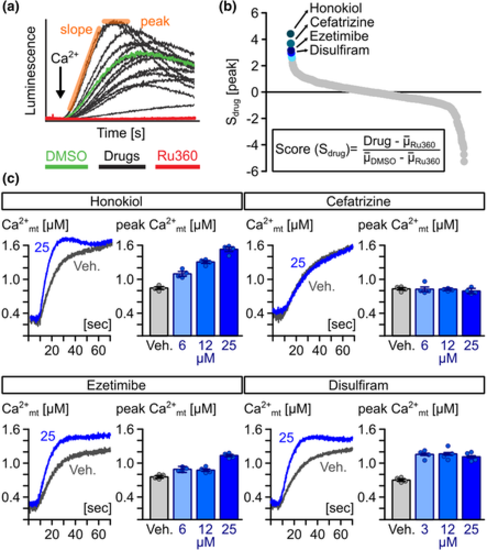
Identification of novel MiCUps by chemical screening. (a) Example of Ca2+-dependent, mitochondrial aequorin kinetics in digitonin-permeabilized HeLa cells upon the addition of 4-μM free Ca2+ (black arrow) in the presence of candidate drugs (10 μM), experimental (10-μM Ru360) and vehicle controls (0.1% [v/v] DMSO). (b) Ranking of compounds based on Sdrug scores. (c) Representative traces of averaged mitochondrial Ca2+ kinetics and quantification of mitochondrial (mt)-Ca2+ uptake rate in mitochondria of permeabilized HeLa cells treated with different concentrations of honokiol, cefatrizine, ezetimibe and disulfiram and 0.1% (v/v) DMSO (vehicle; Veh.) (n = 4)
|
|
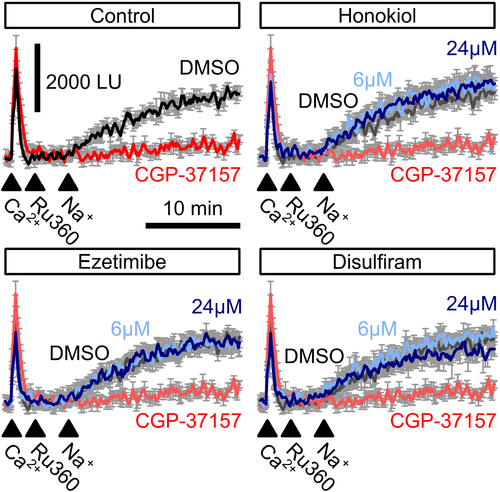
Mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial Ca2+ extrusion. Free Ca2+ in the supernatant of permeabilized HeLa cells was recorded using Calcium Green-5N before and after injection of 100-μM Ca2+ (first arrowhead) followed by a block of mitochondrial Ca2+ uniporter using Ru360 (second arrowhead). Addition of 60-mM Na+ (third arrowhead) induced mitochondrial Na+/Ca2+ exchanger-mediated Ca2+ extrusion (control, black trace) which could be blocked by the mitochondrial Na+/Ca2+ exchanger blocker CGP-37157 (control, red trace). Neither ezetimibe, nor disulfiram or honokiol affected NCLX activity (n = 4)
|
|
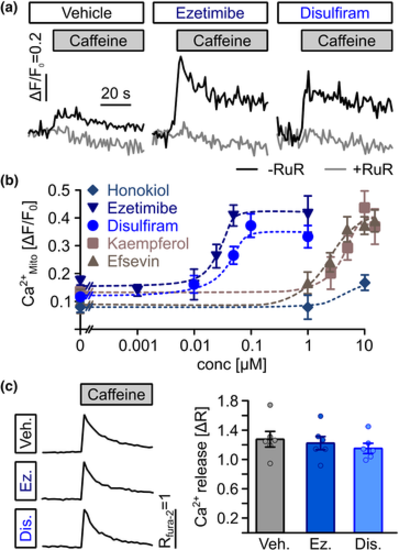
Direct transfer of Ca2+ from the sarcoplasmic reticulum into mitochondria in cardiomyocytes. (a) Representative recordings of mitochondrial (mt)-Ca2+ uptake (black line) in permeabilized HL-1 cardiomyocytes. Superfusion with 10-mM caffeine induced uptake of Ca2+ released from the SR into mitochondria, which was enhanced by 1-μM ezetimibe and 1-μM disulfiram. Ruthenium red as a blocker of RyR, VDAC and mitochondrial Ca2+ uniporter was used to block SR-mitochondria Ca2+ transfer as a negative control. (b) Ezetimibe and disulfiram enhanced SR-mitochondria (Mito) Ca2+ transfer dose dependently from 0.17 ± 0.01 (n = 26 biological replicates from nine experiments) in vehicle-treated control cells to maximum values of ΔF/F0 = 0.41 ± 0.04 at 50 nM for ezetimibe (n = 19 replicates from seven experiments) and from 0.15 ± 0.01 (n = 20 replicates from seven experiments) to 0.37 ± 0.04 at 100 nM for disulfiram (n = 9 replicates from nine individual experiments) and at significantly lower concentrations then the established MiCUps kaempferol and efsevin (ezetimibe: EC50 = 25.5 nM, disulfiram: EC50 = 36.8 nM, kaempferol: EC50 = 3.7 μM, efsevin: EC50 = 2.9 μM), while no significant effect of honokiol could be observed. (c) Ezetimibe (Ez) and disulfiram (Dis) did not alter SR Ca2+ release as assessed by fura-2 fluorescence in intact HL-1 cardiomyocytes after addition of 10-mM caffeine. The baseline fura-2 fluorescence ratio (R340nm/380nm) was 0.75 ± 0.03 for vehicle-treated cells, 0.72 ± 0.03 for cells treated with 1-μM ezetimibe and 0.79 ± 0.03 for cells treated with 1-μM disulfiram. Release of Ca2+ from the SR induced a change in fluorescence (ΔR) of 1.07 ± 0.09 for vehicle-treated cells, 1.02 ± 0.08 for cells treated with 1-μM ezetimibe and 0.96 ± 0.06 for cells treated with 1-μM disulfiram (n = 34 replicates from six experiments, ANOVA)
|
|
Restoration of rhythmic cardiac contractions in the zebrafish arrhythmia model tremblor. (a) Representative confocal linescan recordings through atria of beating GFP-labelled hearts from living zebrafish embryos (TG (myl7:eGFP)) at 1 day after fertilization. Rhythmic cardiac contractions can be observed in wild-type but not tremblor (tre) embryos. Treatment of tre embryos with 1 μM ezetimibe of 1 μM disulfiram restored rhythmic cardiac contractions. (b) Quantification of the percentage of tre embryos with synchronized contractions revealed a rescue of the tre phenotype from 11.1 ± 2.04% embryos with synchronized contractions in the vehicle control (n = 1289 embryos in 15 individual experiments) to 28.39 ± 3.9% of tre embryos at 0.05 μM (n = 155 embryos in six individual experiments), 44.07 ± 7.21% (n = 448 embryos in five individual experiments) after addition of 0.1 μM and a maximum of 53.85 ± 3.38% at 1 μM ezetimibe (n = 354 embryos in five individual experiments). While 0.1 μM disulfiram did not induce a significant effect (n = 507 embryos in five individual experiments), 1 μM disulfiram enhanced the percentage of embryos with synchronized contractions from 9.66 ± 2.31% (n = 1133 embryos from 12 individual experiments) to 54.27 ± 9.48% at 1 μM (n = 371 embryos from five individual experiments) and to 62.83 ± 5.6% at 5 μM (n = 418 embryos from five individual experiments) (Kruskal–Wallis test). (c) Treatment of zebrafish embryos with ezetimibe did not alter gross morphology of embryos, while disulfiram induced severe malformations of the body and a lack of pigmentation
|
|
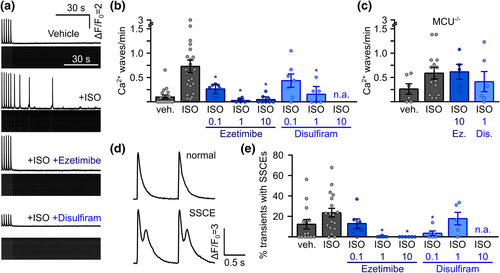
Suppression of arrhythmogenesis in freshly isolated murine RyR2C4496R/WT cardiomyocytes by ezetimibe and disulfiram. (a) Representative confocal linescan recordings of intracellular Ca2+ in freshly isolated ventricular cardiomyocytes from RyR2C4496R/WT mice. Cells were continuously pulsed at 0.5 Hz and spontaneous Ca2+ waves were analysed during 1.5 min after pulsing was stopped. Addition of isoprenaline (ISO) induced spontaneous Ca2+ waves which could be blocked by the addition of ezetimibe or disulfiram. (b) Quantitative analysis of the experiments in (a). Addition of ISO raised the propensity for spontaneous Ca2+ waves from 0.10 ± 0.04 waves per minute (n = 143 cells from 18 mice) to 0.73 ± 0.13 (n = 222 cells from 24 mice) in RyR2C4496R/WT mice. Addition of MiCups lowered waves to 0.27 ± 0.06 under 0.1 μM ezetimibe (n = 64 cells from seven mice), 0.032 ± 0.02 under 1 μM ezetimibe (n = 35 cells from five mice) and, finally, 0.05 ± 0.03 under 10 μM ezetimibe (n = 80 cells from eight mice) and to 0.44 ± 0.14 under 0.1 (n = 49 cells from seven mice) and 0.15 ± 0.08 under 1 (n = 35 cells from six mice) for disulfiram (Kruskal–Wallis test). (c) Disruption of mitochondrial Ca2+ uptake by genetic ablation of mitochondrial Ca2+ uniporter (MCU) in MCU−/− /RyR2C4496R/WT mice abolished the effect of ezetimibe (Ez) and disulfiram (Dis) indicated by comparable values of 0.61 ± 0.15 (n = 42 cells from five mice, P > 0.05 Kruskal–Wallis-test) for ISO + 10 μM ezetimibe and 0.39 ± 0.20 (n = 47 cells from six mice, P > 0.05, Kruskal–Wallis-test) for ISO + 1 μM disulfiram compared to 0.59 ± 0.12 (n = 107 from 14 mice) under ISO alone. (d) Representative recording from electrically induced systolic Ca2+ transients showing normal transients (upper trace) and transients with secondary systolic Ca2+ elevations (SSCEs). (e) SSCEs were observed in 12.4 ± 4.7% (n = 121 cells from 14 mice) of all transients in the vehicle control and rose to 23.3 ± 4.4% after addition of ISO (n = 134 cells from 18 mice). Addition of ezetimibe dose-dependently suppressed SSCEs to a minimum of 0.0% under 10 μM ezetimibe (n = 53 cells from six mice). Disulfiram decreased SSCEs to 3.4 ± 2.2% at 0.1 μM (n = 42 cells from six mice), while cells treated with 1 μM showed SSCEs in 17.5 ± 6.5% of all transients (n = 32 cells from five mice, Kruskal–Wallis test). n.a. = not analysable
|
|
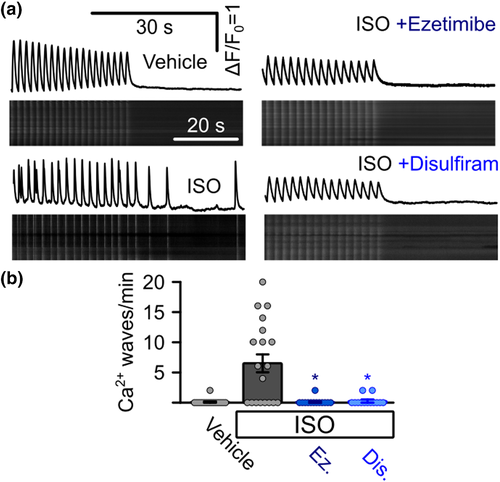
Suppression of arrhythmogenic Ca2+ signals in iPSC-derived cardiomyocytes from a catecholaminergic polymorphic ventricular tachycardia (CPVT) patient. (a) Representative confocal linescan recordings of intracellular Ca2+ in iPSC-derived cardiomyocytes from a CPVT patient. Cells were continuously pulsed at 0.5 Hz and spontaneous Ca2+ waves were analysed after pulsing was stopped. Addition of isoprenaline (ISO) induced spontaneous Ca2+ waves which could be blocked by the addition of ezetimibe or disulfiram. (b) Quantitative analysis of the experiments in (a). Addition of ISO induced the occurrence of waves in CPVT cells (0 waves per minute for vehicle control, n = 11 cells, 5.87 ± 1.80 under ISO, n = 16, Kruskal–Wallis test), which could be suppressed by the addition of ezetimibe (Ez) or disulfiram (Dis) (0.10 ± 0.07 waves per minute for ezetimibe, n = 13 cells, 0.05 ± 0.05 waves per minute for disulfiram, n = 13, Kruskal–Wallis test, data from four independent enzymatic dissociations of 19 individual beating cell clusters)
|