- Title
-
Thyroid hormones regulate the formation and environmental plasticity of white bars in clownfishes
- Authors
- Salis, P., Roux, N., Huang, D., Marcionetti, A., Mouginot, P., Reynaud, M., Salles, O., Salamin, N., Pujol, B., Parichy, D.M., Planes, S., Laudet, V.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Formation of white bars of A. percula new recruits is differentially influenced by age depending on the anemone species. (A and B) Histograms representing percentage of new recruits having 1, 2, or 3 white bars depending on their age in new recruits living in H. magnifica (A) or S. gigantea (B). Statistical tests were done using χ2 tests at each age between H. magnifica or S. gigantea and show statistical difference at 150 to 200 and 200 to 250 dph (respective P = 0.0032 and 0.0011). (C) Number of bars (85% CI) depending on age of individuals predicted from full averaging of the model candidates (D). Blue and orange represent respectively A. percula new recruits sampled in H. magnifica and in S. gigantea. The dots are observed data and are shifted around their number of bars for graphical representation. Predicted regressions of the number of bars are presented for the reference level “lagoon 0.” (D) Full model averaged estimates (85% CI) of linear regression parameters from models including age for each anemone species. The parameter estimates after model averaging of treatment were compared with “Lagoon 1” as reference for the geographic zone. A parameter estimate whose 85% CI includes zero is considered uncertain, and parameter estimates whose 85% CI do not overlap are considered different. |
|
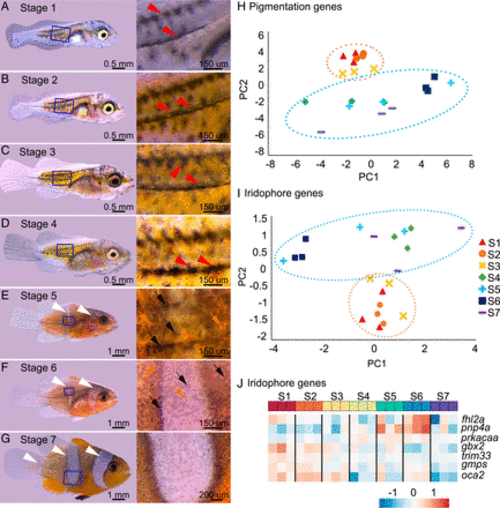
Adult color pattern formation in A. ocellaris is linked to a switch in expression of pigment cells–specific genes during postembryonic development. (A and B) Stereomicroscope images of entire larvae and the associated zoom of the trunk at stage 1 (A), 2 (B), 3 (C), 4 (D), 5 (E), 6 (F), and 7 (G) (adapted from ref. 25). The white and red arrowheads point to white bars and black stripes and black and orange arrows point respectively to melanophores and xanthophores. (H and I) Principal component analysis (PCA) analysis of the pigmentation genes (H) and iridophores genes (I) expression from transcriptomic analysis from entire larvae over postembryonic stages. The two PCA exhibit a clear separation between stages 1 to 3 and stages 4 to 7. The ellipses were arbitrarily drawn around arrays to help resolution: stages 1 to 3 (orange) and 4 to 7 (blue) arrays. All stages had 3 replicates. (J) Heatmap of the seven iridophore genes having the highest fold change between stages 1 to 3 and stages 5 to 7. The color represents the intensity of the centered (but unscaled) signal that goes, for each gene, from low (blue) to medium (white) to high (red). |
|
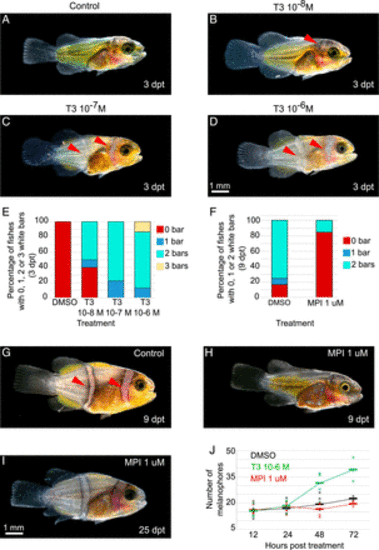
White bars in A. ocellaris form earlier and later, respectively, after treatments with TH or goitrogens. (A–D) Stereomicroscope images of larvae treated at stage 3 during 3 d (dpt) in DMSO (A) or T3 at 10−6 (B), 10−7 (C), and 10−8 M (D). (E and F) Histogram showing the percentage of larvae having 0 (red), 1 (blue), 2 (turquoise), or 3 (yellow) white bars. (E) Larvae are treated at stage 3 for 3 d with DMSO, T3 10−6, 10−7, and 10−8 M (nDMSO = 16, nT3 10−8 M = 20, nT3 10−7 M = 18, nT3 10−6 M = 15 individuals). χ2 tests are significant between T3 10−6 M and DMSO (P < 0.0001). (F) Larvae are treated at stage 3 for 9 d with DMSO or MPI 1 μM (nDMSO = 12, nMPI 1 μM= 13 individuals). Statistical test was done using χ2 tests (P < 0.0029). (G–I) Stereomicroscope images of larvae treated at stage 3 during 9 d in DMSO (G) and MPI 1 μM (H) and MPI 1 μM stage 3 larvae treated for 25 d (I). (J) Graphic showing the number of melanophores in a specific area of the trunk in DMSO (black), T3 10−6 M (green), and MPI 1 μM (red) at 12, 24, 48, and 72 hpt (nDMSO > 9, nT3 > 9, nMPI > 9 individuals). The statistical tests were done using ANOVA between the T3 or MPI treatments and DMSO (control) at each time. The tests are significant between T3 and DMSO at 48 hpt and 72 hpt (P are respectively equal to 0.0299 and 0.0043). The bars correspond to the mean, and crosses correspond to one experiment. hpt = hours posttreatment (scale bar, 1 mm). |
|
duox requirement for the timing of color pattern formation in zebrafish. (A) Graph representing T3 level (in pg of T3 normalized by the weight of the fish in g) in A. percula new recruits sampled in H. magnifica or S. gigantea (nonparametric Mann–Whitney U test, P = 0.0022). (B) Volcano plot of differentially expressed genes between A. percula new recruits living in H. magnifica or S. gigantea. Positive Log2FC values correspond to an increased expression in recruits from S. gigantea, while negative Log2FC corresponds to increased expression in recruits from H. magnifica. The blue and yellow points correspond to significantly differentially expressed genes. The vertical black lines delimit the Log2FC threshold of 1, while the horizontal line corresponds to the corrected P threshold. (C) Inverted fluorescence images show iridophores (dark cells) marked by pnp4a:mem-mCherry expression at 10.6-mm SL in wild-type (Left) and duox CRISPR/Cas9 mutants of zebrafish D. rerio (Right). (D) Numbers of interstripes were scored qualitatively over SL in wild-type (blue, n = 61) and duox CRISPR/Cas9 zebrafish mutants (yellow, n = 51). Complete interstripes received a score of 1 and developing interstripes received a score of 0.5. Each circle represents a single individual and points are jittered vertically for clarity, and equivalently smoothed splines are shown for ease of visualization. The differences in total numbers of interstripes and tractories of interstripe addition resulted in significant effects of genotype (likelihood ratio test, χ2 = 91.7, P < 0.0001, degrees of freedom [d.f.] = 1) and genotype × SL interaction (χ2 = 21.9, P < 0.0001, d.f. = 1). (E) Despite having fewer interstripes overall, duox-deficient zebrafish had proportionally more of the flank covered by dense, interstripe iridophores as compared to the wild type (F1,43 = 76.1, P < 0.0001). The bars indicate means ± 95% CIs (scale bar in A, 200 µm). |