- Title
-
Inflammation Regulates the Multi-Step Process of Retinal Regeneration in Zebrafish
- Authors
- Nagashima, M., Hitchcock, P.F.
- Source
- Full text @ Cells
|
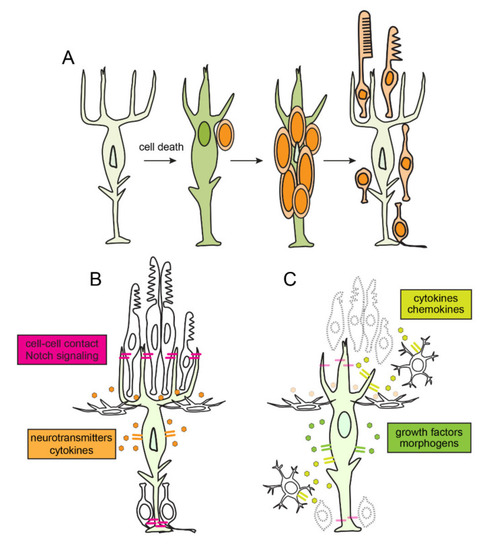
Retinal regeneration, regulation of quiescence and reprogramming of Müller glia in zebrafish. (A) Time course of retinal regeneration. Following cell death, Müller glia dedifferentiate and reprogram into a stem cell-like state. Müller glia then undergo interkinetic nuclear migration an asymmetric self-renewing division, which produces a multipotent retinal progenitor. Müller glia-derived progenitors then proliferate rapidly, migrate and differentiate into the ablated retinal neurons. (B) In an unlesioned retina, cell-cell contact mediated signaling, neurotransmitter dynamics, and autocrine signaling mechanisms maintain Müller glia in a quiescent state. (C) In response to cell death, soluble factors, cytokines, and chemokines, secreted from dying neurons and activated microglia induce reprograming of Müller glia. Müller glia themselves secrete growth factors and morphogens which promote reprogramming in autocrine manner. |
|
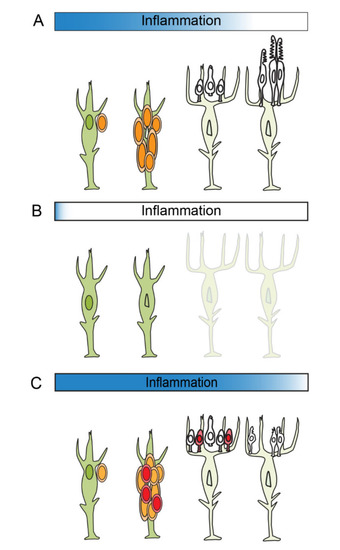
Model of how inflammation regulates photoreceptor regeneration in zebrafish. (A) Following cell death, acute inflammation stimulates reprogramming and cell division among Müller glia and proliferation of Müller glia-derive progenitors. As inflammation resolves, Müller glia-derived progenitors exit the cell cycle and differentiate into photoreceptors. (B) Suppression of acute inflammation results in the failure of Müller glia to proliferate. (C) If acute inflammation is prolonged, Müller glia-derived progenitors undergo extra rounds of cell division. If resolution of acute inflammation fails, this compromises the maturation and survival of photoreceptors. |