|
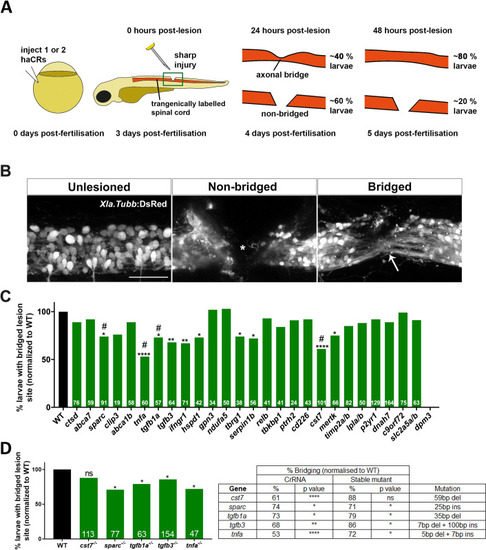
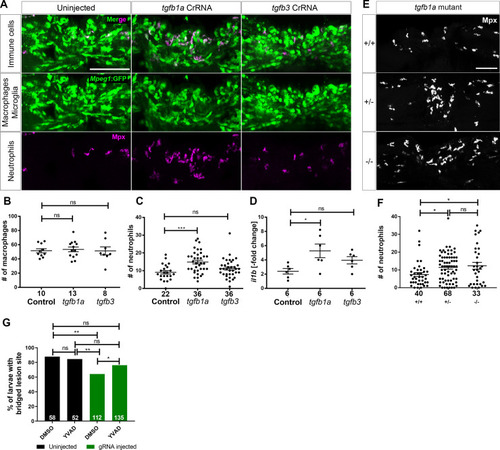
Loss of <italic>tgfb1a</italic> leads to prolonged inflammation.A: Lateral views of lesion sites in larval zebrafish are shown with the indicated markers and experimental conditions at 48 hpl. B-C: Quantifications show that numbers of macrophages were not altered by injecting any of the indicated haCRs (B; one-way ANOVA with Bonferroni’s multiple comparison test; Theoretical power = 0.85 to see a similar increase as for neutrophils in C), neutrophils were increased in number in tgf1b haCRs injected animals (one-way ANOVA with Bonferroni’s multiple comparison test, P = 0.0006), but not in tgfb3 haCRs injected animals (p = 0.32). D: Animals injected with tgfb1a haCRs, but not those injected with tgfb3 haCRs (P = 0.36), displayed marked increases in il1b expression levels in the lesion site compared to lesioned controls at 48 hpl (one-way ANOVA with Bonferroni’s multiple comparison test, P = 0.0211). All transcript levels were normalized to uninjected, unlesioned controls. E-F:tgfb1a heterozygous (one-way ANOVA with Tukey’s multiple comparison test, P = 0.0497) and homozygous mutant animals (P = 0.039) show increased numbers of neutrophils, comparable to haCR-injected animals. G: Inhibition of Il-1β with YVAD rescued axonal bridging compared to the DMSO-treated control group in animals injected with tgfb1a haCRs (Fisher’s exact test * p <0.05, ** p<0.01). Numbers in B, C, F indicate numbers of animals; in D numbers of independent experiments. Error bars represent standard error of the mean (SEM). Scale bars = 50 μm.
EXPRESSION / LABELING:
|
Gene:
|
|
|
Fish:
|
|
|
Condition:
|
|
|
Knockdown Reagents:
|
|
|
Anatomical Term:
|
|
|
Stage:
|
Day 5
|
PHENOTYPE:
|
Fish:
|
|
|
Conditions:
|
|
|
Knockdown Reagents:
|
|
| Observed In: |
|
|
Stage:
|
Day 5
|
|