- Title
-
HDL biodistribution and brain receptors in zebrafish, using HDLs as vectors for targeting endothelial cells and neural progenitors
- Authors
- Sulliman, N.C., Ghaddar, B., Gence, L., Patche, J., Rastegar, S., Meilhac, O., Diotel, N.
- Source
- Full text @ Sci. Rep.
|
|
|
|
|
Relationship between |
|
|
|
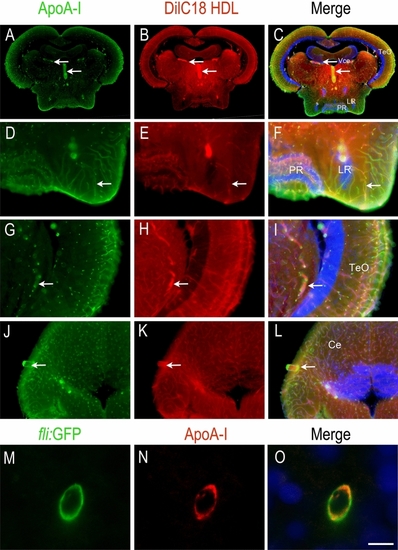
HDLs reach the brain microvasculature in zebrafish. ApoA-I immunohistochemistry (green) on zebrafish brain Sects. 1 h 30 min after intraperitoneal injection with 80 mg/kg of fluorescent HDL particles (red). Note that similar data were obtained with HDLs isolated from plasma (plHDL, n = 3) and with reconstituted HDLs (rHDL, n = 7). ( |
|
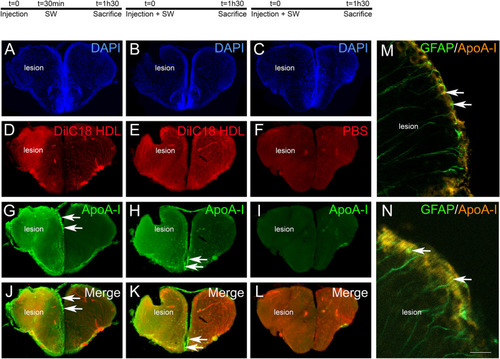
HDLs are taken up by the lesioned hemisphere after stab wound injury in zebrafish. ApoA-I immunohistochemistry (green) on injured zebrafish brain following intraperitoneal injection with 80 mg/kg of red fluorescent reconstituted HDLs (n = 8) or PBS (n = 6) as control. The design of the experiment is presented at the top of the figure. ( |
|
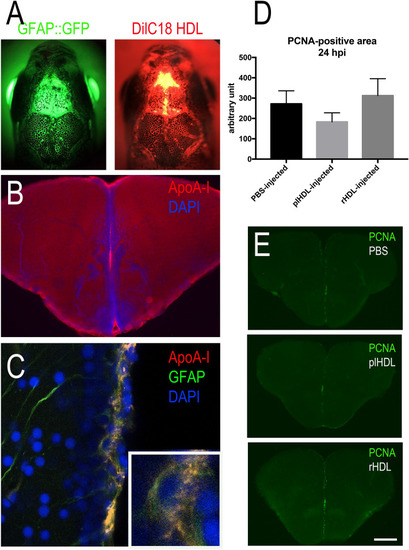
Intracerebroventricular injection of HDLs results in their uptake by neural stem cells but did not modify their proliferation. ( |
|
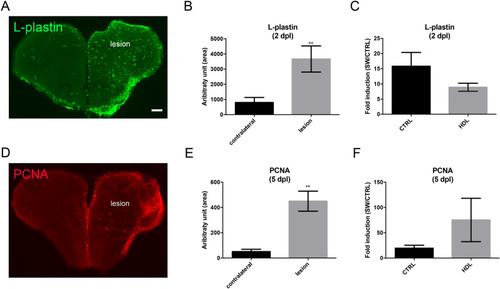
HDL injection did not impact microglia recruitment and injury-induced proliferation at the ventricular zone after telencephalic injury. Zebrafish underwent stab wound injury of the telencephalon and were injected with 80 mg/kg of plasma HDL (n = 9) or PBS as a control (n = 10) and sacrificed at 2- or 5-days post lesion (dpl). ( |
|
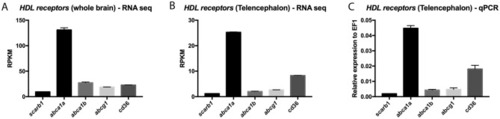
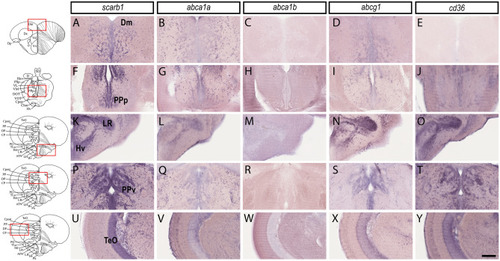
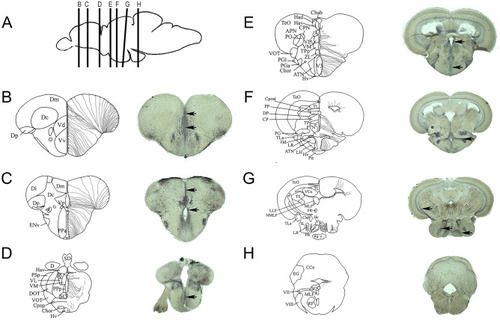
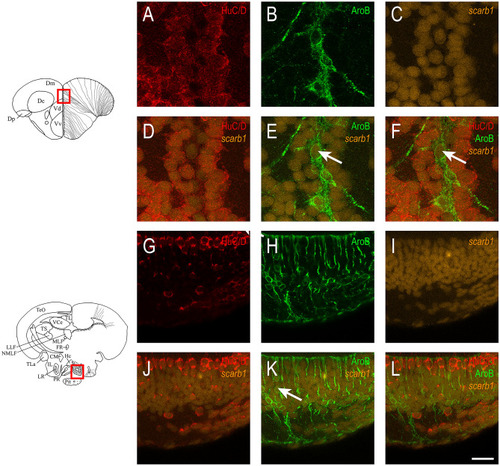
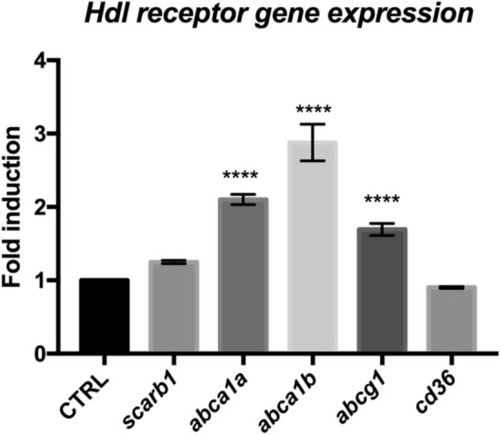
HDL receptors are upregulated at 5 days post brain injury. Reanalysis of the RNA sequencing data set from zebrafish of injured and uninjured telencephalon at 5 days post-lesions (data from |
|
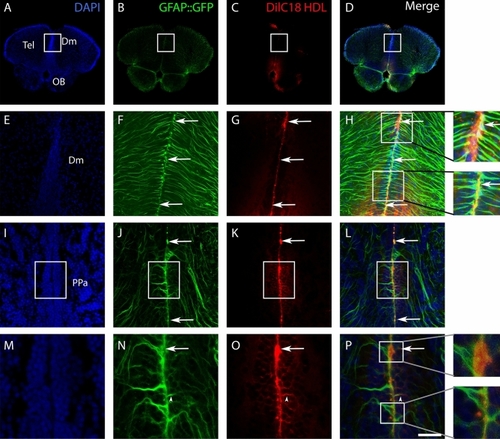
Delivery of DilC18 dye in GFAP::GFP-positive neural stem cells mediated by HDL intracerebroventricular injection. Three adult transgenic zebrafish (GFAP::GFP) were intracerebroventricularly injected with HDLs in which DilC18 dye was incorporated (fish were allowed to survive for 1 h 30 min). ( |