- Title
-
Telomerase reverse transcriptase (TERT) activates transcription of miR500A to inhibit Hedgehog signaling and promote cell invasiveness
- Authors
- Bernabé-García, M., Martínez-Balsalobre, E., García-Moreno, D., García-Castillo, J., Revilla-Nuin, B., Blanco-Alcaina, E., Mulero, V., Alcaraz-Pérez, F., Cayuela, M.L.
- Source
- Full text @ Mol. Oncol.
|
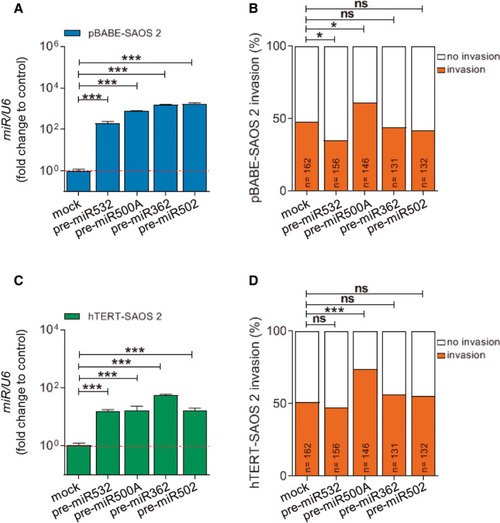
TERT up-regulates the expression of miR500A, leading to an increase in the in vivo invasive capacity. (A) Quantification of the in vivo invasive capacity and (B) proliferation rate of SAOS 2 cells. (C) Quantification of miR500A levels in TERT overexpression conditions by real-time RT-qPCR. (D-G) Overexpression and inhibition of miR500A by transient transfection with the pre-miR500A (D, E) or with an anti-miR500A PNA probe (F, G), respectively, in both pBABE- and hTERT-SAOS 2 cells. (D, F) Quantification of miR500A level and (E, G) its effect on the in vivo invasive capacity. In (C, D, F), each bar represents the mean ± SEM from triplicate samples. In (A, E, G), histogram represents the accumulative value of the invasion percentage of the number of larvae stated in the figure for each treatment. Graphs are representative of three (N = 3) (C, D, F). ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 according to Student's t-test (C), ANOVA followed by Tukey's multiple comparison test (D, F) and Fisher's exact test (A, E, G). |
|
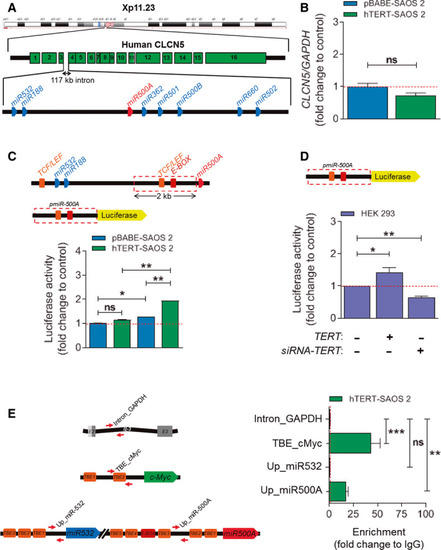
TERT regulates |
|
The |
|
Only the |
|
Telomerase activity is not involved in |
|
The Hedgehog signalling pathway is regulated by |
|
Extracurricular mechanism of TERT during invasion and tumour progression as seen by regulating |