- Title
-
Suppression of Inflammation Delays Hair Cell Regeneration and Functional Recovery Following Lateral Line Damage in Zebrafish Larvae
- Authors
- Zhang, R., Liu, X., Li, Y., Wang, M., Chen, L., Hu, B.
- Source
- Full text @ Biomolecules
|
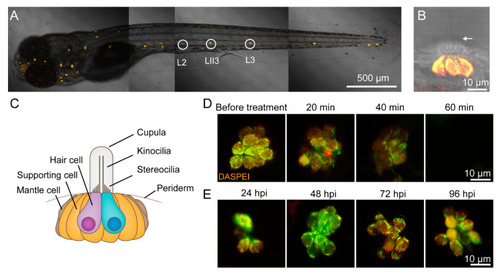
CuSO4 damages hair cells in the lateral line of zebrafish. ( |
|
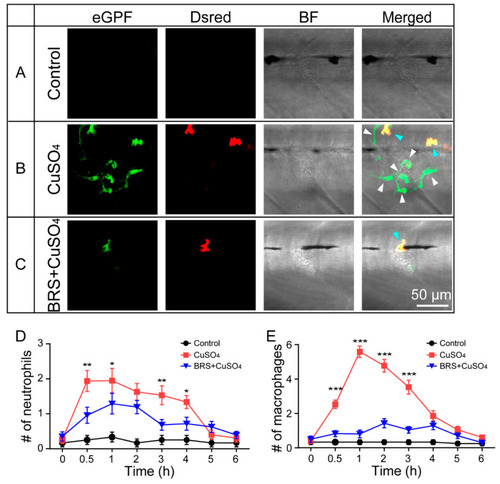
BRS-28 reduces the number of neutrophils and macrophages migrating to the injured neuromasts. ( |
|
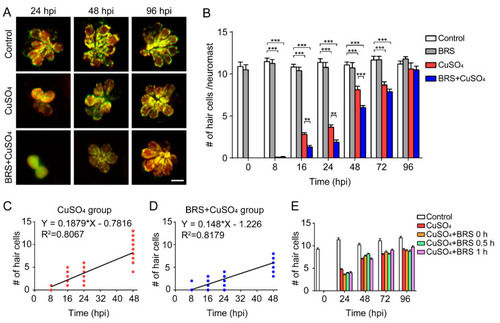
Suppressing inflammation delays hair cell regeneration. ( |
|
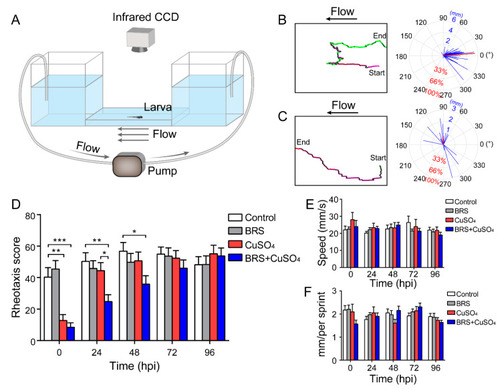
Assessment of rheotaxis reflects the function of the lateral line system. ( |
|
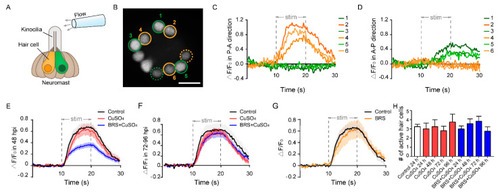
Calcium imaging reveals the function of a single neuromast. ( |