- Title
-
Edible additive effects on zebrafish cardiovascular functionality with hydrodynamic assessment
- Authors
- Wang, Y.F., Chen, I.W., Subendran, S., Kang, C.W., Panigrahi, B., Fu, T.F., Chen, C.Y.
- Source
- Full text @ Sci. Rep.
|
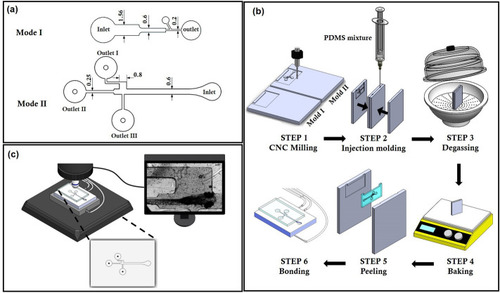
Design, fabrication, and the experimental test set-up of the microfluidic device to assist the hydrodynamic assessment of cardiovascular and the behavioral functionalities in zebrafish. ( |
|
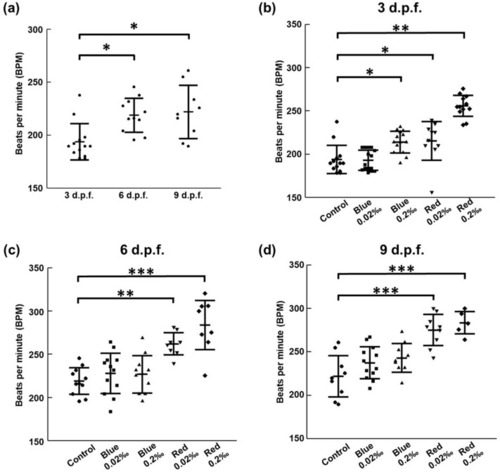
Assessment of the cardiac rhythm of zebrafish larvae treated with food additives. ( |
|
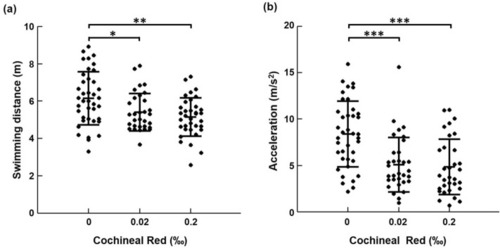
The effects of food additive concentration on the behavioral changes of zebrafish. ( |
|
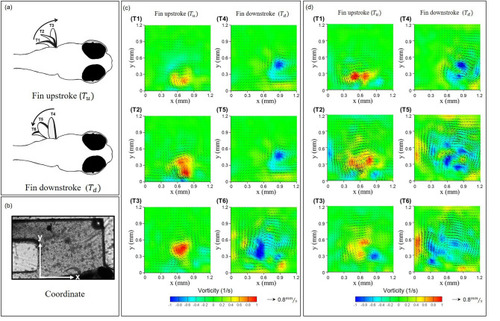
Hydrodynamic quantificational measures of zebrafish pectoral fin movements ( |
|
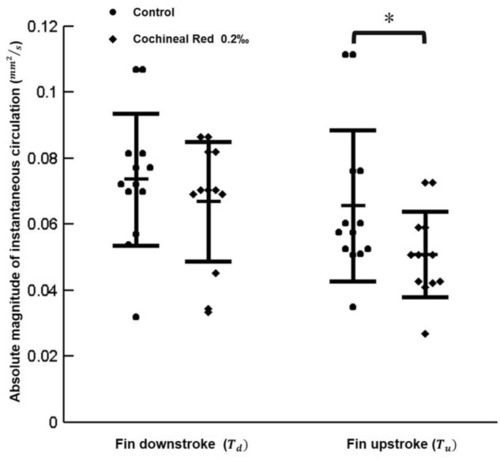
The absolute magnitude and instantaneous circulation formation due to the pectoral fin beating of zebrafish larvae (*p < 0.05) hydrodynamically with effective test samples in each experimental group (N = 15). |