- Title
-
A vertebrate model to reveal neural substrates underlying the transitions between conscious and unconscious states
- Authors
- Bedell, V.M., Meng, Q.C., Pack, M.A., Eckenhoff, R.G.
- Source
- Full text @ Sci. Rep.
|
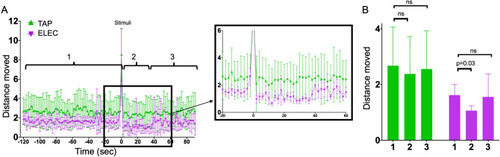
Zebrafish movement before and after TAP and ELEC. ( |
|
Hill plots of the inhaled and IV anesthetics. Zebrafish respond to inhaled anesthetics, halothane ( |
|
Ketamine hill plots and absorption. ( |
|
Hill plots of a sedative drug. Zebrafish respond to dexmedetomidine at physiological concentrations for loss of SPONT ( |
|
Electrical stimulation apparatus made to create a low amplitude, consistent noxious stimulus. ( |