- Title
-
The Zebrafish Amygdaloid Complex - Functional Ground Plan, Molecular Delineation, and Everted Topology
- Authors
- Porter, B.A., Mueller, T.
- Source
- Full text @ Front. Neurosci.
|
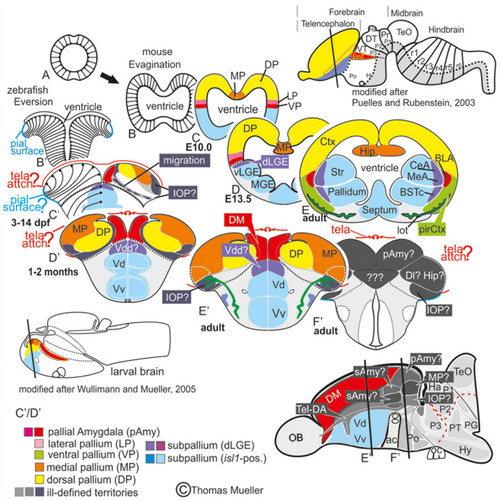
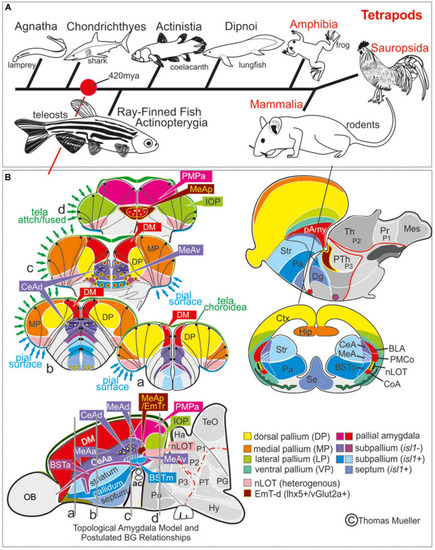
Telencephalic eversion in zebrafish and comparison to mammals. The schematic illustrates how both the outward-growing (eversion) process of the developing telencephalon and its adult morphology of zebrafish (lower row) compares to the telencephalon development (evagination) of mammals (upper row). |
|
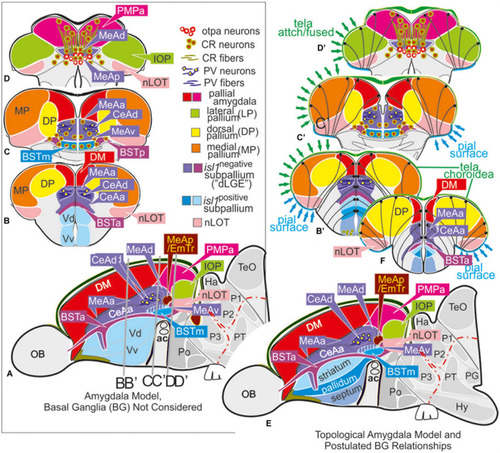
Molecular Definitions of the Zebrafish Amygdaloid Complex. Precommissural |
|
Molecular code of the zebrafish amygdaloid complex. Definitions of amygdaloid territories in the complexly everted telencephalon: Amygdala model (basal ganglia (BG) not considered) |
|
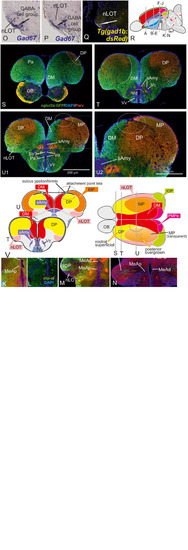
Substance P Fiber Tracts from the Olfactory Bulb into the Telencephalon. |
|
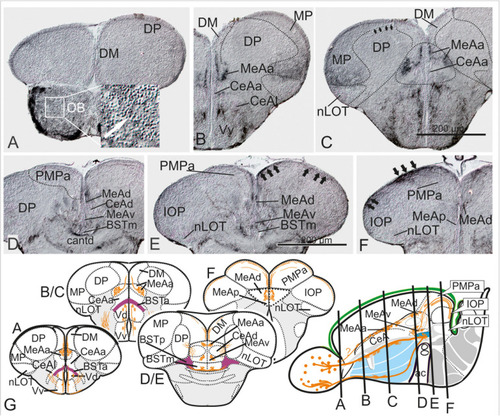
Radial glia cells and Tela Attachement Sites Confirm Medial, Dorsal, and Thalamic Eminence Identification. We performed triple fluorescence immunostains against parvalbumin (PV), GFP, and GFAP in the brains of Tg(vGlut2a:GFP) counterstained against DAPI. |
|
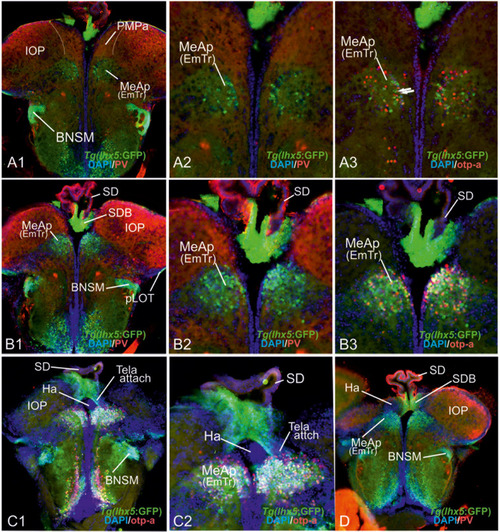
The posterior medial amygdala (MeAp) is identical with the rostral thalamic eminence (EmTr). |
|
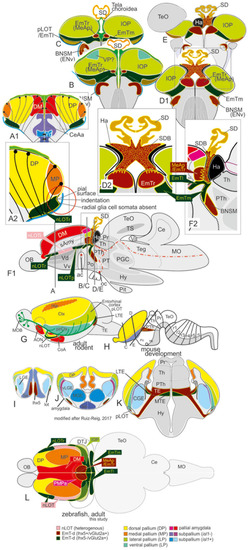
Comparison Zebrafish – Macrosmatic Rodent Amygdala. |
|
Summary schematic explaining how the zebrafish thalamiceminence (EmT) reveals ancestral topological relationships to posterior medial amygdala and olfactory pallium. |