- Title
-
Blood Flow Forces in Shaping the Vascular System: A Focus on Endothelial Cell Behavior
- Authors
- Campinho, P., Vilfan, A., Vermot, J.
- Source
- Full text @ Front. Physiol.
|
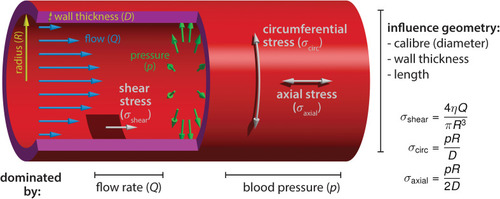
Blood flow-derived forces. Schematic representation of the mechanical forces experienced by endothelial cells due to blood circulation inside the vessels. The blood flow, measured by the volume flow rate |
|
Endothelial cell behaviors triggered by flow-derived mechanical cues during vascular network growth and development. |
|
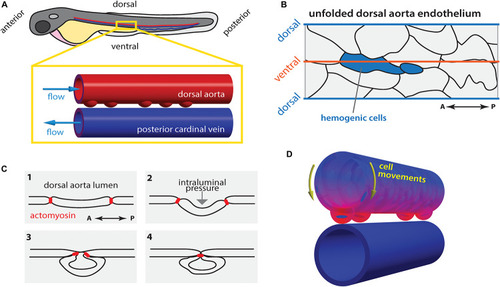
Hematopoietic stem cell emergence is blood flow dependent. |