- Title
-
Lefty blocks a subset of TGFbeta signals by antagonizing EGF-CFC coreceptors
- Authors
- Cheng, S.K., Olale, F., Brivanlou, A.H., Schier, A.F.
- Source
- Full text @ PLoS Biol.
|
|
|
Live wild-type zebrafish embryos at 30 h postfertilization (hpf). (A1, B1, and C1) Ventral views of the head. (A1′, B2, B3, and C1′) Lateral views, with anterior to the left, dorsal up. (A1, A1′, B1, B2, and B3) Wild-type embryos were injected with 20 pg of (C1 and C1′) Wild-type embryos injected with 200 pg of Misexpression of Lefty1 results in cyclopia and other head and trunk mesoderm defects ([A1 and A1′] 32 of 32 embryos had the phenotype shown; arrow shows cyclopia). Coexpression of Cripto with Lefty in embryos leads to rescue of two eyes ([B1] four of 50; arrows show two eyes), notochord ([B2] 20 of 50; inset shows trunk somites and notochord, red bar delineates notochord), and trunk somites ([B3] 50 of 50). Embryos injected with |
|
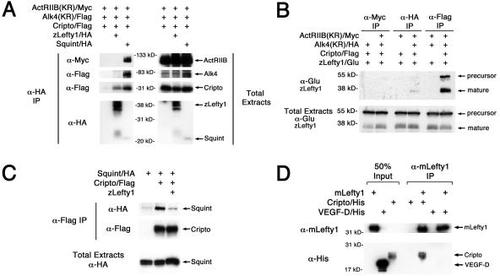
(A and B) Lefty1 interacts with Cripto. RNAs (1 ng each) encoding ActRIIB(KR)/Myc, Alk4(KR)/Flag, Cripto/Flag, Lefty1/HA, or Sqt/HA were injected into (C) Lefty1 competes with Nodal for binding to Cripto. RNAs encoding Sqt/HA (1 ng), Cripto/Flag (100 pg), or Lefty1 (2 ng) were injected and anti-Flag antibody was used to immunopreciptate Cripto/Flag. (D) mLefty1 binds directly to Cripto. Purified mouse Lefty1 protein (mLefty1; 10 μg/ml) was mixed with either soluble purified Cripto/His protein (5 μg/ml) or purified control VEGF-D/His protein (5 μg/ml). After chemical cross-linking, mLefty1 was immunoprecipitated with anti-mLefty1 antibody. mLefty1 associates with Cripto, but not with control VEGF-D. Proteins in the coimmunoprecipitates and total extracts were probed in Western blot analysis with the indicated antibodies: ActRIIB(KR)/Myc (kinase-defective receptor, approximately 120 kDa; anti-Myc), Alk4(KR)/Flag (kinase-defective receptor, approximately 70 kDa; anti-Flag), Cripto/Flag (approximately 30 kDa; anti-Flag), Lefty1/HA (mature ligand, approximately 36–40 kDa; anti-HA; |
|
Schematic depiction of chimeras of mature ligand domains, Finger 1 (F1), Heel (H), and Finger 2 (F2), between |
|
(A) Sequence alignment of Finger 2 region of EGF-CFC-dependent and EGF-CFC-independent TGFβ ligands. Location of secondary structure elements, β-sheets (β6–β9) and loop, are shown ( (B–E) Synthetic mRNAs (200 pg) encoding chimeras of Finger 2 subregions between (B) SqtActβB[loopβ8β9] and SqtActβB[loopβ8] can induce (C) SqtActβB[β8] can weakly expand (D) Other TGFβs conform to loop-β8 EGF-CFC-independent determinant. Note that (E) Wild-type and MZoep embryos were injected with 5 pg of (F) SqtActβB[loopβ8] can bind to ActRIIB and Alk4 in the absence of EGF-CFC coreceptors. RNAs (1 ng each) encoding ActRIIB(KR)/Myc, Alk4(KR)/Flag, Cripto/Flag, ActivinβB/HA, Sqt/HA, or SqtActβB[loopβ8]/HA were injected into |
|
Synthetic mRNAs (200 pg) encoding Sqt harboring multiple mutations from ActivinβB (shown in red) were injected into wild-type and MZ |
|
Synthetic mRNAs (200 pg) encoding ActivinβB with single or double region substitutions from Sqt were injected into wild-type and MZ |
|
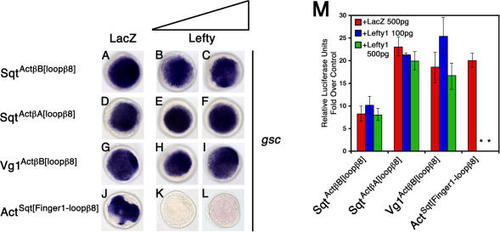
(A–L) Embryos were injected with 75 pg of (M) Wild-type embryos were injected with 75 pg of either SqtActβB[loopβ8], SqtActβA[loopβ8], Vg1ActβB[loopβ8], or 200 pg of ActSqt[Finger1-loopβ8] mRNA. Embryos were further double-injected with 500 pg of |
|
(A) In the absence of ligands, the EGF-CFC coreceptor (solid pink) is constitutively bound to the type I receptor Alk4 (solid green). (B) Nodal (solid blue) binds to receptor complexes consisting of EGF-CFC/Alk4 and ActRIIB (solid green). (C) Lefty (solid yellow) sequesters the EGF-CFC coreceptor, thereby preventing Nodal binding to the receptor complexes. (D) Subtle sequence differences determine the interaction with the EGF-CFC coreceptor and the Lefty inhibitor. Nodal and Vg1/GDF1 (solid blue) require the EGF-CFC coreceptor for signaling through ActRIIB and Alk4, while Activin (solid red) does not. SqtActβB[loopβ8] and Vg1ActβB[loopβ8] (solid blue with red strip) containing the loop-β8 region of ActivinβB can bind to ActRIIB and Alk4 without the EGF-CFC coreceptor and therefore cannot be blocked by Lefty. ActSqt[Finger1-loopβ8] (solid red with two blue strips) requires the coreceptor for receptor complex binding and can be inhibited by Lefty. |