- Title
-
Octominin: A Novel Synthetic Anticandidal Peptide Derived from Defense Protein of Octopus minor
- Authors
- Nikapitiya, C., Dananjaya, S.H.S., Chandrarathna, H.P.S.U., De Zoysa, M., Whang, I.
- Source
- Full text @ Mar. Drugs
|
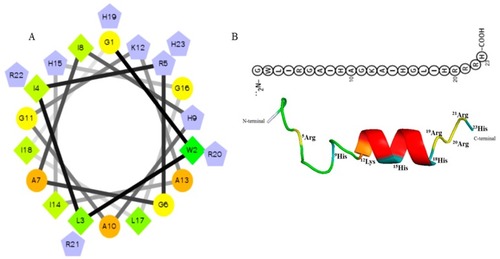
Predicted helical secondary and three-dimensional structures of Octominin. ( |
|
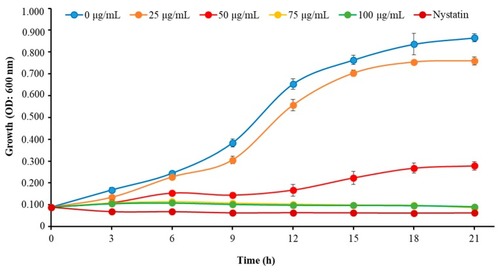
Time–kill kinetics of Octominin against |
|
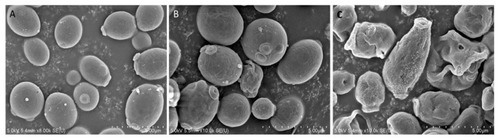
Effect of Octominin on morphological and structural changes of |
|
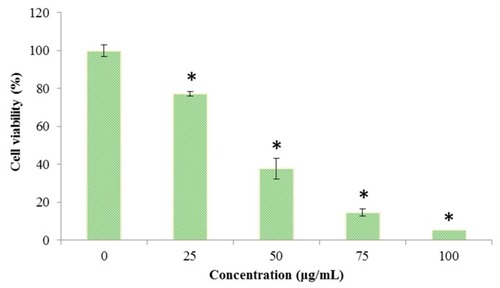
Effect of Octominin on the viability of |
|
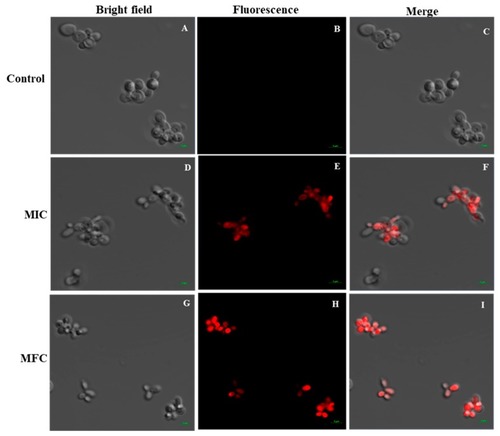
Effect of Octominin on membrane permeability in |
|
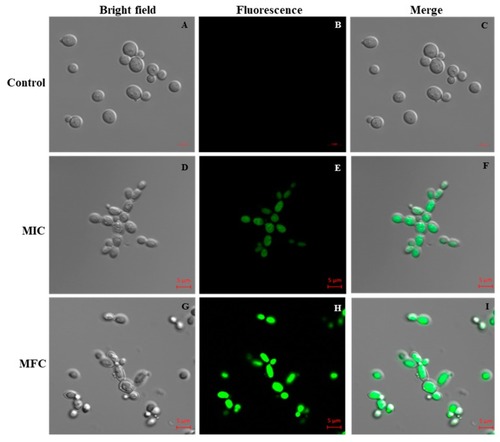
Effect of Octominin on reactive oxygen species (ROS) production of |
|
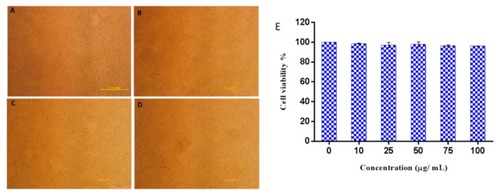
Cytotoxic effect of Octominin on HEK293 cells. ( |
|
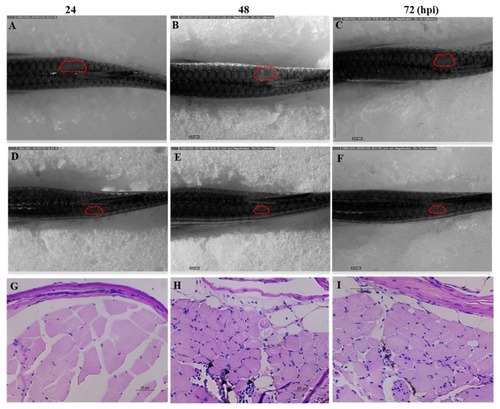
Anticandidal effect of Octominin in PHENOTYPE:
|